Latest recommendations

| Id | Title * | Authors * | Abstract * | Picture * | Thematic fields * ▲ | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
26 Aug 2022
Within and among population differences in cuticular hydrocarbons in the seabird tick Ixodes uriaeMarlène Dupraz, Chloe Leroy, Thorkell Lindberg Thórarinsson, Patrizia d’Ettorre, Karen D. McCoy https://doi.org/10.1101/2022.01.21.477272Seabird tick diversification and cuticular hydrocarbonsRecommended by Felix Sperling based on reviews by 2 anonymous reviewersTicks are notorious vectors of diseases in humans and other vertebrates. Much effort has been expended to understand tick diversity and ecology with the aim of managing their populations to alleviate the misery they bring. Further, the fundamental question of whether ticks are usually host generalists or host specialists has been debated at length and is important both for understanding the mechanisms of their diversification as well as for focusing control of ticks [1]. One elegant resolution of this question is to consider most tick species to be global generalists but local specialists [1]. This is well illustrated in a series of studies of the seabird tick, Ixodes uriae, which is comprised of host-specific races that show genetic [2], morphological [3] and host performance [4] differences associated with the seabirds they feed on. Such a pattern has clear ramifications for sympatric speciation; however, the factors that potentially act to drive these differences have remained elusive. Dupraz et al. [5] have now made intriguing and important steps toward bridging the gap between demonstrating local patterns of tick host association and understanding the physiological mechanisms that may facilitate such divergences. They collected I. uriae ticks from the nests of two seabirds – Atlantic puffins and common guillemots – on the north side of Iceland. Four populations of ticks were sampled, with one island providing both puffin ticks and guillemot ticks, to give two tick populations from each of the two seabird host species. They then washed the ticks in solvent and analyzed the dissolved cuticular hydrocarbons (CHCs) using GC mass spectrometry, revealing 22 different hydrocarbon compounds common to most of these samples. CHCs are known to be important across arthropods for a variety of functions ranging from reducing water loss to facilitating communication and recognition between individuals with species. Dupraz et al. [5] found three hydrocarbons that distinguished puffin ticks most consistently from guillemot ticks. A cross-validation test for host type also assigned 75% of the tick pools to the seabird host of origin. However, with these limited sample sizes, statistical analysis revealed no significant difference in CHC profiles between the host types, although a tendency was evident. Nonetheless, this study revealed a number of potentially diagnostic CHCs for tick host type, as well as some that may be more diagnostic of locations. This provides a fascinating and actionable foundation for further work using additional sites and host types, as well as an entry point into discerning the mechanisms at play in producing the diversity, complexity and adaptability that make ticks such medical menaces. References | Within and among population differences in cuticular hydrocarbons in the seabird tick Ixodes uriae | Marlène Dupraz, Chloe Leroy, Thorkell Lindberg Thórarinsson, Patrizia d’Ettorre, Karen D. McCoy | <p>The hydrophobic layer of the arthropod cuticle acts to maintain water balance, but can also serve to transmit chemical signals via cuticular hydrocarbons (CHC), essential mediators of arthropod behavior. CHC signatures typically vary qualitativ... |  | Acari, Biology, Ecology, Evolution | Felix Sperling | 2022-02-08 13:00:52 | View | |
28 Aug 2022
A simple procedure to detect, test for the presence of stuttering, and cure stuttered data with spreadsheet programsThierry de Meeûs and Camille Noûs https://doi.org/10.5281/zenodo.7029324Improved population genetics parameters through control for microsatellite stutteringRecommended by Michael Lattorff based on reviews by Thibaut Malausa, Fabien Halkett and Thierry Rigaud based on reviews by Thibaut Malausa, Fabien Halkett and Thierry Rigaud
Molecular markers have drastically changed and improved our understanding of biological processes. In combination with PCR, markers revolutionized the study of all organisms, even tiny insects, and eukaryotic pathogens amongst others. Microsatellite markers were the most prominent and successful ones. Their success started in the early 1990s. They were used for population genetic studies, mapping of genes and genomes, and paternity testing and inference of relatedness. Their popularity is based on some of their characteristics as codominance, the high polymorphism information content, and their ease of isolation (Schlötterer 2004). Still, microsatellites are the marker of choice for a range of non-model organisms as next-generation sequencing technologies produce a huge amount of single nucleotide polymorphisms (SNPs), but often at expense of sample size and higher costs. | A simple procedure to detect, test for the presence of stuttering, and cure stuttered data with spreadsheet programs | Thierry de Meeûs and Camille Noûs | <p>Microsatellite are powerful markers for empirical population genetics, but may be affected by amplification problems like stuttering that produces heterozygote deficits between alleles with one repeat difference. In this paper, we present a sim... | Acari, Ecology, Evolution, Genetics/Genomics, Helminthology, Invertebrates, Medical entomology, Molecular biology, Parasitology, Theoretical biology, Veterinary entomology | Michael Lattorff | 2021-12-06 14:30:47 | View | ||
27 Apr 2023
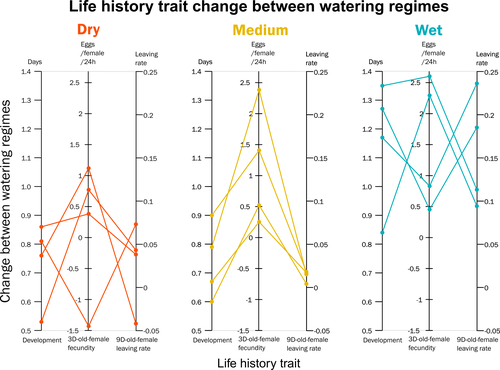
Climate of origin influences how a herbivorous mite responds to drought-stressed host plantsAlain Migeon, Philippe Auger, Odile Fossati-Gaschignard, Ruth A. Hufbauer, Maëva Miranda, Ghais Zriki, Maria Navajas https://doi.org/10.1101/2021.10.21.465244Not all spider-mites respond in the same way to droughtRecommended by Inês Fragata and Raul Costa-Pereira based on reviews by Bastien Castagneyrol and 2 anonymous reviewersBiotic interactions are often shaped by abiotic factors (Liu and Gaines 2022). Although this notion is not new in ecology and evolutionary biology, we are still far from a thorough understanding of how biotic interactions change along abiotic gradients in space and time. This is particularly challenging because abiotic factors can affect organisms and their interactions in multiple – direct or indirect – ways. For example, because abiotic conditions strongly determine how energy enters biological systems via producers, their effects can propagate through entire food webs, from the bottom to the top (O’Connor 2009, Gilbert et al 2019). Understanding how biological diversity - both within and across species - is shaped by the indirect effects of environmental conditions is a timely question as climate change and anthropogenic activities have been altering temperature and water availability across different ecosystems. Motivated by the current water crisis and severe droughts predicted for the near future worldwide (du Plessis 2019), Migeon et al. (2023) investigated how water limitation on producers scales up to affect life-history patterns of a widespread crop pest, the spider mite Tetranychus urticae. The authors sampled spider mite populations (n = 12) along a striking gradient of climatic conditions (>16 degrees of latitude) in Europe. After letting mites acclimate to lab conditions for several generations, the authors performed a common garden experiment to quantify how the life-history traits of mite populations from different locations respond to drought stress in their host plants. Curiously, the authors found that, when reared on drought-stressed plants, mites tended to develop faster, had higher fecundity and lower dispersion rates. This response was in line with some results obtained previously with Tetranychus species (e.g. Ximénez-Embun et al 2016). Importantly, despite some experimental caveats in the experimental design, which makes it difficult to completely disentangle the specific effects of location vs. environmental noise, results suggest the climate that populations originally experienced was also an important determinant of the plastic response in these herbivores. In fact, populations from wetter and colder regions showed a steeper change in drought response, while populations from arid climates showed a shallower response. This interesting result suggests the importance of intraspecific (between-populations) variation in the response to drought, which might be explained by the climatic heterogeneity in space throughout the evolutionary history of different populations. These results become even more important in our rapidly changing world, highlighting the importance of considering genetic variation (and conditions that generate it) when predicting plastic and evolutionary responses to stressful conditions. du Plessis, A. (2019). Current and Future Water Scarcity and Stress. In: Water as an Inescapable Risk. Springer Water. Springer, Cham. https://doi.org/10.1007/978-3-030-03186-2 | Climate of origin influences how a herbivorous mite responds to drought-stressed host plants | Alain Migeon, Philippe Auger, Odile Fossati-Gaschignard, Ruth A. Hufbauer, Maëva Miranda, Ghais Zriki, Maria Navajas | <p style="text-align: justify;">Drought associated with climate change can stress plants, altering their interactions with phytophagous arthropods. Drought not only impacts cultivated plants but also their parasites, which in some cases are favore... |  | Acari, Ecology, Life histories | Inês Fragata | 2021-10-22 14:56:03 | View | |
14 Nov 2023

Time-course of antipredator behavioral changes induced by the helminth Pomphorhynchus laevis in its intermediate host Gammarus pulex: the switch in manipulation according to parasite developmental stage differs between behaviorsThierry Rigaud, Aude Balourdet, Alexandre Bauer https://doi.org/10.1101/2023.04.25.538244Exploring manipulative strategies of a trophically-transmitted parasite across its ontogenyRecommended by Thierry Lefevre based on reviews by Adèle Mennerat and 1 anonymous reviewerThe intricate relationships between parasites and their hosts often involve a choreography of behavioral changes, with parasites manipulating their hosts in a way that enhances - or seemingly enhances – their transmission (Hughes et al., 2012; Moore, 2002; Poulin, 2010). Host manipulation is increasingly acknowledged as a pervasive adaptive transmission strategy employed by parasites, and as such is one of the most remarkable manifestations of the extended phenotype (Dawkins, 1982). In this laboratory study, Rigaud et al. (2023) delved into the time course of antipredator behavioral modifications induced by the acanthocephalan Pomphorhynchus laevis in its amphipod intermediate host Gammarus pulex. This system has a good foundation of prior knowledge (Bakker et al., 2017; Fayard et al., 2020; Perrot-Minnot et al., 2023), nicely drawn upon for the present work. This parasite orchestrates a switch from predation suppression, during the noninfective phase, to predation enhancement upon maturation. Specifically, G. pulex infected with the non-infective acanthella stage of the parasite can exhibit increased refuge use and reduced activity compared to uninfected individuals (Dianne et al., 2011, 2014), leading to decreased predation by trout (Dianne et al., 2011). In contrast, upon reaching the infective cystacanth stage, the parasite can enhance the susceptibility of its host to trout predation (Dianne et al., 2011). The present work aimed to understand the temporal sequence of these behavioral changes across the entire ontogeny of the parasite. The results confirmed the protective role of P. laevis during the acanthella stage, wherein infected amphipods exhibited heightened refuge use. This protective manipulation, however, became significant only later in the parasite's ontogeny, suggesting a delayed investment strategy, possibly influenced by the extended developmental time of P. laevis. The protective component wanes upon reaching the cystacanth stage, transitioning into an exposure strategy, aligning with theoretical predictions and previous empirical work (Dianne et al., 2011; Parker et al., 2009). The switch was behavior-specific. Unlike the protective behavior, a decline in the amphipod activity rate manifested early in the acanthella stage and persisted throughout development, suggesting potential benefits of reduced activity for the parasite across multiple stages. Furthermore, the findings challenge previous assumptions regarding the condition-dependency of manipulation, revealing that the parasite-induced behavioral changes predominantly occurred in the presence of cues signaling potential predators. Finally, while amphipods infected with acanthella stages displayed survival rates comparable to their uninfected counterparts, increased mortality was observed in those infected with cystacanth stages. Understanding the temporal sequence of host behavioral changes is crucial for deciphering whether it is adaptive to the parasite or not. This study stands out for its meticulous examination of multiple behaviors over the entire ontogeny of the parasite highlighting the complexity and condition-dependent nature of manipulation. The protective-then-expose strategy emerges as a dynamic process, finely tuned to the developmental stages of the parasite and the ecological challenges faced by the host. The delayed emergence of protective behaviors suggests a strategic investment by the parasite, with implications for the host's survival and the parasite's transmission success. The differential impact of infection on refuge use and activity rate further emphasizes the need for a multidimensional approach in studying parasitic manipulation (Fayard et al., 2020). This complexity demands further exploration, particularly in deciphering how trophically-transmitted parasites shape the behavioral landscape of their intermediate hosts and its temporal dynamic (Herbison, 2017; Perrot-Minnot & Cézilly, 2013). As we discover the many subtleties of these parasitic manipulations, new avenues of research are unfolding, promising a deeper understanding of the ecology and evolution of host-parasite interactions. References Bakker, T. C. M., Frommen, J. G., & Thünken, T. (2017). Adaptive parasitic manipulation as exemplified by acanthocephalans. Ethology, 123(11), 779–784. https://doi.org/10.1111/eth.12660 Dawkins, R. (1982). The extended phenotype: The long reach of the gene (Reprinted). Oxford University Press. Dianne, L., Perrot-Minnot, M.-J., Bauer, A., Gaillard, M., Léger, E., & Rigaud, T. (2011). Protection first then facilitation: A manipulative parasite modulates the vulnerability to predation of its intermediate host according to its own developmental stage. Evolution, 65(9), 2692–2698. https://doi.org/10.1111/j.1558-5646.2011.01330.x Dianne, L., Perrot-Minnot, M.-J., Bauer, A., Guvenatam, A., & Rigaud, T. (2014). Parasite-induced alteration of plastic response to predation threat: Increased refuge use but lower food intake in Gammarus pulex infected with the acanothocephalan Pomphorhynchus laevis. International Journal for Parasitology, 44(3–4), 211–216. https://doi.org/10.1016/j.ijpara.2013.11.001 Fayard, M., Dechaume‐Moncharmont, F., Wattier, R., & Perrot‐Minnot, M. (2020). Magnitude and direction of parasite‐induced phenotypic alterations: A meta‐analysis in acanthocephalans. Biological Reviews, 95(5), 1233–1251. https://doi.org/10.1111/brv.12606 Herbison, R. E. H. (2017). Lessons in Mind Control: Trends in Research on the Molecular Mechanisms behind Parasite-Host Behavioral Manipulation. Frontiers in Ecology and Evolution, 5, 102. https://doi.org/10.3389/fevo.2017.00102 Hughes, D. P., Brodeur, J., & Thomas, F. (2012). Host manipulation by parasites. Oxford university press. Moore, J. (2002). Parasites and the behavior of animals. Oxford University Press. Parker, G. A., Ball, M. A., Chubb, J. C., Hammerschmidt, K., & Milinski, M. (2009). When should a trophically transmitted parasite manipulate its host? Evolution, 63(2), 448–458. https://doi.org/10.1111/j.1558-5646.2008.00565.x Perrot-Minnot, M.-J., & Cézilly, F. (2013). Investigating candidate neuromodulatory systems underlying parasitic manipulation: Concepts, limitations and prospects. Journal of Experimental Biology, 216(1), 134–141. https://doi.org/10.1242/jeb.074146 Perrot-Minnot, M.-J., Cozzarolo, C.-S., Amin, O., Barčák, D., Bauer, A., Filipović Marijić, V., García-Varela, M., Servando Hernández-Orts, J., Yen Le, T. T., Nachev, M., Orosová, M., Rigaud, T., Šariri, S., Wattier, R., Reyda, F., & Sures, B. (2023). Hooking the scientific community on thorny-headed worms: Interesting and exciting facts, knowledge gaps and perspectives for research directions on Acanthocephala. Parasite, 30, 23. https://doi.org/10.1051/parasite/2023026 Poulin, R. (2010). Parasite Manipulation of Host Behavior. In Advances in the Study of Behavior (Vol. 41, pp. 151–186). Elsevier. https://doi.org/10.1016/S0065-3454(10)41005-0 Rigaud, T., Balourdet, A., & Bauer, A. (2023). Time-course of antipredator behavioral changes induced by the helminth Pomphorhynchus laevis in its intermediate host Gammarus pulex: The switch in manipulation according to parasite developmental stage differs between behaviors. bioRxiv, ver. 6 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.04.25.538244 | Time-course of antipredator behavioral changes induced by the helminth *Pomphorhynchus laevis* in its intermediate host *Gammarus pulex*: the switch in manipulation according to parasite developmental stage differs between behaviors | Thierry Rigaud, Aude Balourdet, Alexandre Bauer | <p style="text-align: justify;">Many trophically transmitted parasites with complex life cycles manipulate their intermediate host antipredatory defenses in ways facilitating their transmission to final host by predation. Some parasites also prote... |  | Aquatic, Behavior, Crustacea, Invertebrates, Parasitology | Thierry Lefevre | 2023-06-20 15:49:32 | View | |
08 Feb 2022

The initial response of females towards congeneric males matches the propensity to hybridise in OphthalmotilapiaMaarten Van Steenberge, Noemie Jublier, Loic Kever, Sophie Gresham, Sofie Derycke, Jos Snoeks, Eric Parmentier, Pascal Poncin, Erik Verheyen https://doi.org/10.1101/2021.08.07.455508Experimental evidence for asymmetrical species recognition in East African Ophthalmotilapia cichlidsRecommended by Ellen Decaestecker based on reviews by George Turner and 2 anonymous reviewersI recommend the Van Steenberge et al. study. With over 2000 endemic species, the East African cichlids are a well-established model system in speciation research (Salzburger 2018) and several models have been proposed and tested to explain how these radiations formed (Kocher 2004). Hybridization was shown to be a main driver of the rapid speciation and adaptive radiations of the East African Cichlid fishes (Seehausen 2004). However, it is obvious that unrestrained hybridization also has the potential to reduce taxonomic diversity by erasing species barriers. In the classical model of cichlid evolution, special emphasis was placed on mate preference (Kocher 2004). However, no attention was placed on species recognition, which was implicitly assumed. There is, however, more research needed on what species recognition means, especially in radiating lineages such as cichlids. In a previous study, Nevado et al. 2011 found traces of asymmetrical hybridization between members of the Lake Tanganyika radiation: the genus Ophthalmotilapia. This recommended study by Van Steenberge et al. is based on Nevado et al. (2011), which detected that in one genus of Ophthalmotilapia mitochondrial DNA ‘typical’ for one of the four species (O. nasuta) was also found in three other species (O. ventralis, O. heterodonta, and O. boops). The authors suggested that this could be explained by the fact that females of the three other species accepted O. nasuta males, but that O. nasuta females were more selective and accepted only conspecifc males. This could hence be due to asymmetric mate preferences, or by asymmetric abilities for species recognition. This is exactly what the current study by Van Steenberge et al. did. They tested the latter hypothesis by presenting females of two different Ophthalmotilapia species with con- and heterospecific males. This was tested through experiments, making use of wild specimens of two species: O. nasuta and O. ventralis. The authors assumed that if they performed classical “choice-experiments”, they would not notice the recognition effects, given that females would just select preferred, most likely conspecific, males. Instead, specimens were only briefly presented to other fishes since the authors wanted to compare differences in the ability for ‘species recognition’. In this, the authors followed Mendelson and Shaw (2012) who used “a measurable difference in behavioural response towards conspecifics as compared to heterospecifics’’ as a definition for recognition. Instead of the focus on selection/preference, they investigated if females of different species behaved differently, and hence detected the difference between conspecific and heterospecific males. This was tested by a short (15 minutes) exposure to another fish in an isolated part of the aquarium. Recognition was defined as the ‘difference in a particular behaviour between the two conditions’. What was monitored was the swimming behaviour and trajectory (1 image per second) together with known social behaviours of this genus. The selection of these behaviours was further facilitated based on experimental set-ups of reproductive behaviour or the same species previously described by the same research team (Kéver et al. 2018). The result was that O. nasuta females, for which it was expected that they would not hybridize, showed a different behaviour towards a con- or a heterospecific male. They interacted less with males of the other species. What was unexpected is that there was no difference in behaviour of the females whether they recognized a male or (control) female of their own species. This suggests that they did not detect differences in reproductive behaviour, but rather in the interactions between conspecifics. For females of O. ventralis, for which there are indications for hybridization in the wild, they did not find a difference in behaviour. Females of this species behaved identically with respect to the right and wrong males as well as towards the control females. Interestingly is thus that a complex pattern between species in the wild could be (partially) explained by the behaviour/interaction at first impression of the individuals of these species. References Kéver L, Parmentier E, Derycke S, Verheyen E, Snoeks J, Van Steenberge M, Poncin P (2018) Limited possibilities for prezygotic barriers in the reproductive behaviour of sympatric Ophthalmotilapia species (Teleostei, Cichlidae). Zoology, 126, 71–81. https://doi.org/10.1016/j.zool.2017.12.001 Kocher TD (2004) Adaptive evolution and explosive speciation: the cichlid fish model. Nature Reviews Genetics, 5, 288–298. https://doi.org/10.1038/nrg1316 Mendelson TC, Shaw KL (2012) The (mis)concept of species recognition. Trends in Ecology & Evolution, 27, 421–427. https://doi.org/10.1016/j.tree.2012.04.001 Nevado B, Fazalova V, Backeljau T, Hanssens M, Verheyen E (2011) Repeated Unidirectional Introgression of Nuclear and Mitochondrial DNA Between Four Congeneric Tanganyikan Cichlids. Molecular Biology and Evolution, 28, 2253–2267. https://doi.org/10.1093/molbev/msr043 Salzburger W (2018) Understanding explosive diversification through cichlid fish genomics. Nature Reviews Genetics, 19, 705–717. https://doi.org/10.1038/s41576-018-0043-9 Seehausen O (2004) Hybridization and adaptive radiation. Trends in Ecology & Evolution, 19, 198–207. https://doi.org/10.1016/j.tree.2004.01.003 Steenberge MV, Jublier N, Kéver L, Gresham S, Derycke S, Snoeks J, Parmentier E, Poncin P, Verheyen E (2022) The initial response of females towards congeneric males matches the propensity to hybridise in Ophthalmotilapia. bioRxiv, 2021.08.07.455508, ver. 3 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2021.08.07.455508 | The initial response of females towards congeneric males matches the propensity to hybridise in Ophthalmotilapia | Maarten Van Steenberge, Noemie Jublier, Loic Kever, Sophie Gresham, Sofie Derycke, Jos Snoeks, Eric Parmentier, Pascal Poncin, Erik Verheyen | <p style="text-align: justify;">Cichlid radiations often harbour closely related species with overlapping niches and distribution ranges. Such species sometimes hybridise in nature, which raises the question how can they coexist. This also holds f... |  | Aquatic, Behavior, Evolution, Fish, Vertebrates, Veterinary entomology | Ellen Decaestecker | 2021-08-09 12:22:49 | View | |
26 Apr 2023
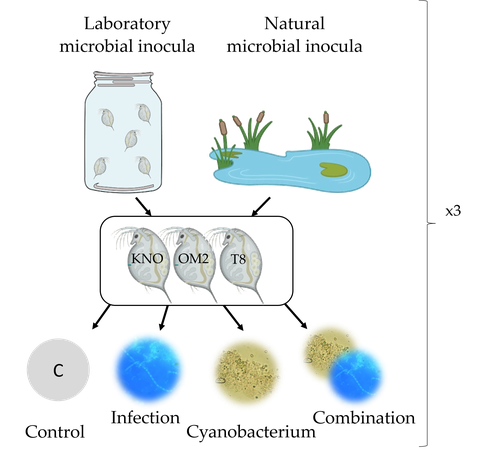
Microbiome mediated tolerance to biotic stressors: a case study of the interaction between a toxic cyanobacterium and an oomycete-like infection in Daphnia magnaShira Houwenhuyse*, Lore Bulteel*, Isabel Vanoverberghe, Anna Krzynowek, Naina Goel, Manon Coone, Silke Van den Wyngaert, Arne Sinnesael, Robby Stoks & Ellen Decaestecker https://doi.org/10.31219/osf.io/9n4mgMulti-stress responses depend on the microbiome in the planktonic crustacean DaphniaRecommended by Bertanne Visser and Mathilde Scheifler based on reviews by Natacha Kremer and 2 anonymous reviewers based on reviews by Natacha Kremer and 2 anonymous reviewers
The critical role that gut microbiota play in many aspects of an animal’s life, including pathogen resistance, detoxification, digestion, and nutritional physiology, is becoming more and more apparent (Engel and Moran 2013; Lindsay et al., 2020). Gut microbiota recruitment and maintenance can be largely affected by the surrounding environment (Chandler et al., 2011; Callens et al., 2020). The environment may thus dictate gut microbiota composition and diversity, which in turn can affect organismal responses to stress. Only few studies have, however, taken the gut microbiota into account to estimate life histories in response to multiple stressors in aquatic systems (Macke et al., 2016). Houwenhuyse et al., investigate how the microbiome affects life histories in response to ecologically relevant single and multiple biotic stressors (an oomycete-like parasite, and a toxic cyanobacterium) in Daphnia magna (Houwenhuyse et al., 2023). Daphnia is an excellent model, because this aquatic system lends itself extremely well for gut microbiota transplantation and manipulation. This is due to the possibility to sterilize eggs (making them free of bacteria), horizontal transmission of bacteria from the environment, and the relative ease of culturing genetically similar Daphnia clones in large numbers. The authors use an elegant experimental design to show that the Daphnia gut microbial community differs when derived from a laboratory versus natural inoculum, the latter being more diverse. The authors subsequently show that key life history traits (survival, fecundity, and body size) depend on the stressors (and combination thereof), the microbiota (structure and diversity), and Daphnia genotype. A key finding is that Daphnia exposed to both biotic stressors show an antagonistic interaction effect on survival (being higher), but only in individuals containing laboratory gut microbiota. The exact mechanism remains to be determined, but the authors propose several interesting hypotheses as to why Daphnia with more diverse gut microbiota do less well. This could be due, for example, to increased inter-microbe competition or an increased chance of contracting opportunistic, parasitic bacteria. For Daphnia with less diverse laboratory gut microbiota, a monopolizing species may be particularly beneficial for stress tolerance. Alongside these interesting findings, the paper also provides extensive information about the gut microbiota composition (available in the supplementary files), which is a very useful resource for other researchers. Overall, this study reveals that multiple, interacting factors affect the performance of Daphnia under stressful conditions. Of importance is that laboratory studies may be based on simpler microbiota systems, meaning that stress responses measured in the laboratory may not accurately reflect what is happening in nature. REFERENCES Callens M, De Meester L, Muylaert K, Mukherjee S, Decaestecker E. The bacterioplankton community composition and a host genotype dependent occurrence of taxa shape the Daphnia magna gut bacterial community. FEMS Microbiology Ecology. 2020;96(8):fiaa128. https://doi.org/10.1093/femsec/fiaa128 Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLOS Genetics. 2011;7(9):e1002272. https://doi.org/10.1371/journal.pgen.1002272 Engel P, Moran NA. The gut microbiota of insects - diversity in structure and function. FEMS Microbiology Reviews. 2013;37(5):699-735. https://doi.org/10.1111/1574-6976.12025 Houwenhuyse S, Bulteel L, Vanoverberghe I, Krzynowek A, Goel N et al. Microbiome mediated tolerance to biotic stressors: a case study of the interaction between a toxic cyanobacterium and an oomycete-like infection in Daphnia magna. 2023. OSF, ver. 2 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.31219/osf.io/9n4mg Lindsay EC, Metcalfe NB, Llewellyn MS. The potential role of the gut microbiota in shaping host energetics and metabolic rate. Journal of Animal Ecology. 2020;89(11):2415-2426. https://doi.org/10.1111/1365-2656.13327 Macke E, Tasiemski A, Massol F, Callens M, Decaestecker E. Life history and eco-evolutionary dynamics in light of the gut microbiota. Oikos. 2017;126(4):508-531. https://doi.org/10.1111/oik.03900 | Microbiome mediated tolerance to biotic stressors: a case study of the interaction between a toxic cyanobacterium and an oomycete-like infection in *Daphnia magna* | Shira Houwenhuyse*, Lore Bulteel*, Isabel Vanoverberghe, Anna Krzynowek, Naina Goel, Manon Coone, Silke Van den Wyngaert, Arne Sinnesael, Robby Stoks & Ellen Decaestecker | <p style="text-align: justify;">Organisms are increasingly facing multiple, potentially interacting stressors in natural populations. The ability of populations coping with combined stressors depends on their tolerance to individual stressors and ... |  | Aquatic, Biology, Crustacea, Ecology, Life histories, Symbiosis | Bertanne Visser | 2021-05-17 16:18:18 | View | |
03 Jul 2020

The 'Noble false widow' spider Steatoda nobilis is an emerging public health and ecological threatHambler, C. https://doi.org/10.31219/osf.io/axbd4How the noble false widow spider Steatoda nobilis can turn out to be a rising public health and ecological concernRecommended by Etienne Bilgo based on reviews by Michel Dugon and 2 anonymous reviewers"The noble false widow spider Steatoda nobilis is an emerging public health and ecological threat" by Clive Hambler (2020) is an appealing article discussing important aspects of the ecology and distribution of a medically significant spider, and the health concerns it raises. References [1] Hambler, C. (2020). The “Noble false widow” spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints, axbd4, ver. 4 peer-reviewed and recommended by PCI Zoology. doi: 10.31219/osf.io/axbd4 | The 'Noble false widow' spider Steatoda nobilis is an emerging public health and ecological threat | Hambler, C. | <p>*Steatoda nobilis*, the 'Noble false widow' spider, has undergone massive population growth in southern Britain and Ireland, at least since 1990. It is greatly under-recorded in Britain and possibly globally. Now often the dominant spider on an... |  | Arachnids, Behavior, Biogeography, Biological invasions, Conservation biology, Demography/population dynamics, Ecology, Medical entomology, Methodology, Pest management, Toxicology, Veterinary entomology | Etienne Bilgo | 2019-06-28 18:26:05 | View | |
30 Nov 2022
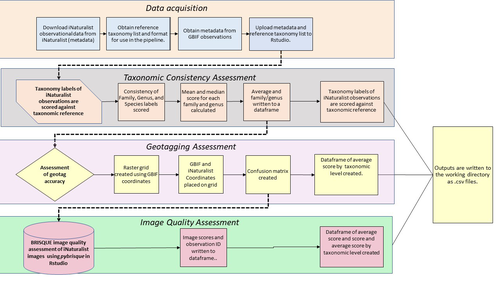
A pipeline for assessing the quality of images and metadata from crowd-sourced databases.Jackie Billotte https://doi.org/10.1101/2022.04.29.490112Harnessing the full potential of iNaturalist and other databasesRecommended by Matthias Foellmer based on reviews by Clive Hambler and Catherine ScottThe popularity of iNaturalist and other online biodiversity databases to which the general public and specialists alike contribute observations has skyrocketed in recent years (Dance 2022). The AI-based algorithms (computer vision) which provide the first identification of a given organism on an uploaded photograph have become very sophisticated, suggesting initial identifications often down to species level with a surprisingly high degree of accuracy. The initial identifications are then confirmed or improved by feedback from the community, which works particularly well for organismal groups to which many active community members contribute, such as the birds. Hence, providing initial observations and identifying observations of others, as well as browsing the recorded biodiversity for given locales or the range of occurrences of individual taxa has become a meaningful and satisfying experience for the interested naturalist. Furthermore, several research studies have now been published relying on observations uploaded to iNaturalist (Szentivanyi and Vincze 2022). However, using the enormous amount of natural history data available on iNaturalist in a systematic way has remained challenging, since this requires not only retrieving numerous observations from the database (in the hundreds or even thousands), but also some level of transparent quality control. Billotte (2022) provides a protocol and R scripts for the quality assessment of downloaded observations from iNaturalist, allowing an efficient and reproducible stepwise approach to prepare a high-quality data set for further analysis. First, observations with their associated metadata are downloaded from iNaturalist, along with the corresponding entries from the Global Biodiversity Information Facility (GBIF). In addition, a taxonomic reference list is obtained (these are available online for many taxa), which is used to assess the taxonomic consistency in the dataset. Second, the geo-tagging is assessed by comparing the iNaturalist and GBIF metadata. Lastly, the image quality is assessed using pyBRISQUE. The approach is illustrated using spiders (Araneae) as an example. Spiders are a very diverse taxon and an excellent taxonomic reference list is available (World Spider Catalogue 2022). However, spiders are not well known to most non-specialists, and it is not easy to take good pictures of spiders without using professional equipment. Therefore, the ability of iNaturalist’s computer vision to provide identifications is limited to this date and the community of specialists active on iNaturalist is comparatively small. Hence, spiders are a good taxon to demonstrate how the pipeline results in a quality-controlled dataset based on crowed-sourced data. Importantly, the software employed is free to use, although inevitably, the initial learning curve to use R scripts can be steep, depending on prior expertise with R/RStudio. Furthermore, the approach is employable with databases other than iNaturalist. In summary, Billotte's (2022) pipeline allows researchers to use the wealth of observations on iNaturalist and other databases to produce large metadata and image datasets of high-quality in a reproducible way. This should pave the way for more studies, which could include, for example, the assessment of range expansions of invasive species or the evaluation of the presence of endangered species, potentially supporting conservation efforts. References Billotte J (2022) A pipeline for assessing the quality of images and metadata from crowd-sourced databases. BiorXiv, 2022.04.29.490112, ver 5 peer reviewed and recommended by Peer Community In Zoology. https://doi.org/10.1101/2022.04.29.490112 Dance A (2022) Community science draws on the power of the crowd. Nature, 609, 641–643. https://doi.org/10.1038/d41586-022-02921-3 Szentivanyi T, Vincze O (2022) Tracking wildlife diseases using community science: an example through toad myiasis. European Journal of Wildlife Research, 68, 74. https://doi.org/10.1007/s10344-022-01623-5 World Spider Catalog (2022). World Spider Catalog. Version 23.5. Natural History Museum Bern, online at http://wsc.nmbe.ch. https://doi.org/10.24436/2 | A pipeline for assessing the quality of images and metadata from crowd-sourced databases. | Jackie Billotte | <p style="text-align: justify;">Crowd-sourced biodiversity databases provide easy access to data and images for ecological education and research. One concern with using publicly sourced databases; however, is the quality of their images, taxonomi... |  | Arachnids, Biodiversity, Biology, Conservation biology, Ecology, Insecta, Invertebrates | Matthias Foellmer | 2022-05-03 00:18:23 | View | |
20 Dec 2022

Non-target effects of ten essential oils on the egg parasitoid Trichogramma evanescensLouise van Oudenhove, Aurélie Cazier, Marine Fillaud, Anne-Violette Lavoir, Hicham Fatnassi, Guy Pérez, Vincent Calcagno https://doi.org/10.1101/2022.01.14.476310Side effects of essential oils on pest natural enemiesRecommended by Cedric Pennetier based on reviews by Olivier Roux and 2 anonymous reviewersIntegrated pest management relies on the combined use of different practices in time and/or space. The main objectives are to better control pests, not to induce too much selective pressure on resistance mechanisms present in pest populations and to minimize non-targeted effects on the ecosystem [1]. The efficiency of such a strategy requires at least additional or synergistic effects of chosen tools against targeted pest population in a specific environment. Any antagonistic effect on targeted or non-targeted organisms might reduce control effort to nil even worst. Van Oudenhove et al [2] raised the question of the interaction between botanical pesticides (BPs) and egg parasitoids. Each of these two strategies used for pest management present advantages and are described as eco-friendly. First, the use of parasitoids is a great example of biological control and is massively used in a broad range of crop production in different ecological settings. Second, BPs, especially essential oils (EOs) used for a wide range of activities on pests (repellent, antifeedant, antiovipositant, ovicidal, larvicidal and simply pesticidal) present low-toxicity to non-target vertebrates and do not last too long in the environment. Combining these two strategies might be considered as a great opportunity to better pest control with minimized impact on environment. However, EOs used to target a wide range of pest might directly or indirectly affect parasitoids. Van Oudenhove et al [2] focused their study on non-target effects of 10 essentials oils with pesticide potential on larval development and egg-seeking behaviour of five strains of the biocontrol agent Trichogramma evanescens. Within two laboratory experiments mimicing EOs fumigation (i.e. contactless EOs exposure), the authors evaluated (1) the toxicity of EOs on parasitoid development and (2) the repellent effect of these EOs on adult wasps. They confirmed that contactless exposure of EOs can (1) induce mortality during pre-imaginal development (more acute at the pupal stage) and (2) induce behavioural avoidance of EOs odour plume. These experiments ran onto five strains of T. evanescens also highlighted the variation of the effects of EOs among parasitoid strains. The complex and dynamic interaction between pest, plant, parasitoid (a natural enemy) and their environment is disturbed by EOs. EOs plumes are also dynamic and variable upon the environmental conditions. The results of van Oudenhove et al. experimentally illustrate such a complexity by describing opposite effects (repellent and attractive) of the same EO on the behaviour of two T. evanescens strains. These contrasting results led us to question more broadly the non-target effects of pest management programs based on EOs fumigation on natural enemies. Finally, the limits of this experimental study as discussed in the paper draw research avenues taking into account biotic variables such as plant chemical cues, odour plume dynamics, individual behavioural experiences and abiotic variables such as temperature, light and gravity [3] in laboratory, semi-field and field experiments. Facing such a complexity, modelling studies at fine scale in time and space have the operational objective to help farmers to choose the best IPM strategy regarding their environment (as illustrated for aphid population management in the recent review by Stell et al. [4]). But before such research effort to be undertaken, Van Oudenhove et al study [2] sounds like an alert for a cautious use of EOs in pest control programs that integrate biological control with parasitoids.
References [1] Fauvergue, X. Biocontrôle Elements Pour Une Protection Agroecologique des Cultures; Éditions Quae: Versailles, France, 2020. [2] van Oudenhove L, Cazier A, Fillaud M, Lavoir AV, Fatnassi H, Pérez G, Calcagno V. Non-target effects of ten essential oils on the egg parasitoid Trichogramma evanescens. bioRxiv 2022.01.14.476310, ver. 4 peer-reviewed and recommended by PCI Zoology. https://doi.org/10.1101/2022.01.14.476310 [3] Victor Burte, Guy Perez, Faten Ayed, Géraldine Groussier, Ludovic Mailleret, Louise van Oudenhove and Vincent Calcagno (2022) Up and to the light: intra- and interspecific variability of photo- and geo-tactic oviposition preferences in genus Trichogramma, Peer Community Journal, 2: e3. https://doi.org/10.24072/pcjournal.78 [4] Stell E, Meiss H, Lasserre-Joulin F, Therond O. Towards Predictions of Interaction Dynamics between Cereal Aphids and Their Natural Enemies: A Review. Insects 2022, 13, 479. https://doi.org/10.3390/insects13050479 | Non-target effects of ten essential oils on the egg parasitoid Trichogramma evanescens | Louise van Oudenhove, Aurélie Cazier, Marine Fillaud, Anne-Violette Lavoir, Hicham Fatnassi, Guy Pérez, Vincent Calcagno | <p style="text-align: justify;">Essential oils (EOs) are increasingly used as biopesticides due to their insecticidal potential. This study addresses their non-target effects on a biological control agent: the egg parasitoid <em>Trichogramma evane... |  | Behavior, Biochemistry, Biocontrol, Biodiversity, Computer modelling, Conservation biology, Demography/population dynamics, Development, Ecology, Insecta, Insectivores, Invertebrates, Life histories, Methodology, Pest management, Theoretical biolo... | Cedric Pennetier | 2022-01-31 16:05:32 | View | |
25 Aug 2021
Up and to the light: intra- and interspecific variability of photo- and geo-tactic oviposition preferences in genus TrichogrammaBurte, V., Perez, G., Ayed, F. , Groussier, G., Mailleret, L., van Oudenhove, L. and Calcagno, V. https://doi.org/10.1101/2021.03.30.437671New insights into oviposition preference of 5 Trichogramma speciesRecommended by Joël Meunier based on reviews by Kévin Tougeron and Eveline C. Verhulst based on reviews by Kévin Tougeron and Eveline C. Verhulst
Insects exhibit a great diversity of life-history traits that often vary not only between species but also between populations of the same species (Flatt and Heyland, 2011). A better understanding of the variation in these traits can be of paramount importance when it comes to species of economic and agricultural interest (Wilby and Thomas, 2002). In particular, the control of the development and expansion of agricultural pests generally requires a good understanding of the parameters that favour the reproduction of these pests and/or the reproduction of the species used to control them (Bianchi et al., 2013; Gäde and Goldsworthy, 2003). Parasitoid wasps of the genus Trichogramma are a classic example of insects involved in pest control (Smith, 1996). This genus comprises over 200 species worldwide, which have been used to control populations of a wide range of lepidopteran pests since the 1900s (Flanders, 1930; Hassan, 1993). Despite its common use, the egg-laying preference of this genus is only partially known. For example, all Trichogramma species are often thought to have positive phototaxis (or negative geotaxis) (e.g. Brower & Cline, 1984; van Atta et al., 2015), but comprehensive studies simultaneously testing this (or other) parameter among Trichogramma species and populations remain rare. This is exactly the aim of the present study (Burte et al., 2021). Using a new experimental approach based on automatic image analysis, the authors compared the photo- and geo-tactic oviposition preference among 5 Trichogramma species from 25 populations. Their results first confirm that most Trichogramma species and populations prefer light to shade, and higher to lower positions for oviposition. Interestingly, they also reveal that the levels of preference for light and gravity show inter- and intraspecific variation (probably due to local adaptation to different strata) and that both preferences tend to relax over time. Overall, this study provides important information for improving the use of Trichogramma species as biological agents. For example, it may help to establish breeding lines adapted to the microhabitat and/or growing parts of plants on which agricultural pests lay eggs most. Similarly, it suggests that the use of multiple strains with different microhabitat selection preferences could lead to better coverage of host plants, as well as a reduction in intraspecific competition in the preferred parts. Finally, this study provides a new methodology to efficiently and automatically study oviposition preferences in Trichogramma, which could be used in other insects with a particularly small size. References Bianchi, F. J. J. A., Schellhorn, N. A. and Cunningham, S. A. (2013). Habitat functionality for the ecosystem service of pest control: reproduction and feeding sites of pests and natural enemies. Agricultural and Forest Entomology, 15, 12–23. https://doi.org/10.1111/j.1461-9563.2012.00586.x Burte V., Perez G., Ayed F., Groussier G., Mailleret L, van Oudenhove L. and Calcagno V. (2021). Up and to the light: intra- and interspecific variability of photo-and geo-tactic oviposition preferences in genus Trichogramma. bioRxiv, 2021.03.30.437671, ver. 4 peer-reviewed and recommended by PCI Zoology. https://doi.org/10.1101/2021.03.30.437671 Brower, J. H. and Cline, L. D. (1984). Response of Trichogramma pretiosum and T. evanescens to Whitelight, Blacklight or NoLight Suction Traps. The Florida Entomologist, 67, 262–268. https://doi.org/10.2307/3493947 Flanders, S. E. (1930). Mass production of egg parasites of the genus Trichogramma. Hilgardia, 4, 465–501. https://doi.org/10.3733/hilg.v04n16p465 Flatt, T. and Heyland, A. (2011). Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199568765.001.0001 Gäde, G. and Goldsworthy, G. J. (2003). Insect peptide hormones: a selective review of their physiology and potential application for pest control. Pest Management Science, 59, 1063–1075. https://doi.org/10.1002/ps.755 Hassan, S. A. (1993). The mass rearing and utilization of Trichogramma to control lepidopterous pests: Achievements and outlook. Pesticide Science, 37, 387–391. https://doi.org/10.1002/ps.2780370412 Smith, S. M. (1996). Biological Control with Trichogramma : Advances, Successes, and Potential of Their Use. Annual Review of Entomology, 41, 375–406. https://doi.org/10.1146/annurev.en.41.010196.002111 van Atta, K. J., Potter, K. A. and Woods, H. A. (2015). Effects of UV-B on Environmental Preference and Egg Parasitization by Trichogramma Wasps (Hymenoptera: Trichogrammatidae). Journal of Entomological Science, 50, 318–325. https://doi.org/10.18474/JES15-09.1 Wilby, A. and Thomas, M. B. (2002). Natural enemy diversity and pest control: patterns of pest emergence with agricultural intensification. Ecology Letters, 5, 353–360. https://doi.org/10.1046/j.1461-0248.2002.00331.x | Up and to the light: intra- and interspecific variability of photo- and geo-tactic oviposition preferences in genus Trichogramma | Burte, V., Perez, G., Ayed, F. , Groussier, G., Mailleret, L., van Oudenhove, L. and Calcagno, V. | <p>Trichogramma are parasitic microwasps much used as biological control agents. The genus is known to harbor tremendous diversity, at both inter- and intra-specific levels. The successful selection of Trichogramma strains for biocontrol depends o... |  | Behavior, Biocontrol, Biodiversity, Ecology, Insecta, Parasitology, Pest management, Systematics, Terrestrial | Joël Meunier | Kévin Tougeron, Eveline C. Verhulst | 2021-04-02 16:10:28 | View |
MANAGING BOARD
Dominique Adriaens
Ellen Decaestecker
Benoit Facon
Isabelle Schon
Emmanuel Toussaint
Bertanne Visser










