Latest recommendations

| Id | Title * | Authors * | Abstract * ▼ | Picture * | Thematic fields * | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
31 Jul 2024
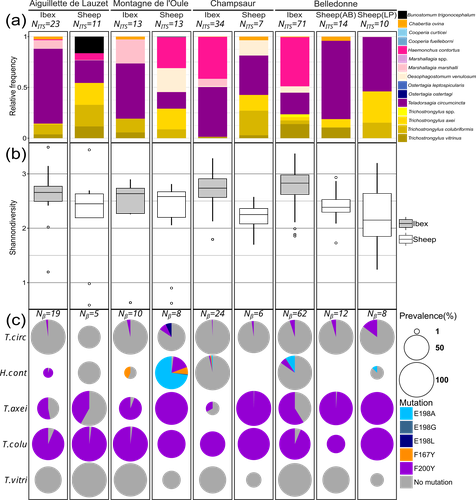
Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheepCamille Beaumelle, Carole Toigo, Rodolphe Papet, Slimania Benabed, Mathieu Beurier, Lea Bordes, Anais Brignone, Nadine Curt-Grand-Gaudin, Mathieu Garel, Justine Ginot, Philippe Jacquiet, Christian Miquel, Marie-Therese Poirel, Anna Serafino, Eric Vannard, Gilles Bourgoin, Glenn Yannic https://doi.org/10.1101/2023.07.21.550073What gets left behind? Shared nematode communities at the wildlife-livestock interface.Recommended by Karen D McCoy based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
Gastrointestinal nematodes represent a major problem for livestock production across the globe, one that has intensified with the rapid and repeated evolution of multi-drug resistance (Wit et al., 2021). Understanding parasite exposure and how resistance is maintained over time are therefore of key importance for defining efficient management strategies. To date, the role wildlife play in these dynamics has been poorly studied. The work of Beaumelle et al. examine this essential question by studying the transmission dynamics of nematodes at the environmental interface between transhumant sheep and wild ungulates, more specifically with ibex (Capra ibex) that allochronically share alpine pastures when sheep are brought to graze in summer. By collecting fresh fecal material from both species and using a metabarcoding approach based on ITS-2 sequences, the authors characterise the nemabiome in each ungulate species and demonstrate that the two host species share a large portion of their parasite diversity. More importantly, by focusing on a gene (β-tubulin isotype 1) associated with resistance to a commonly used anthelmintic drug (benzimidazole), they demonstrate that both species carry resistant nematode strains, but that the diversity of strains, and particularly susceptible strains, is much higher in ibex. A key feature of the sampling design is that fecal material from both species was collected before seasonal transmission between the ungulate species could occur. Therefore, their results demonstrate that ibex are able to maintain resistant strains over long periods of time and therefore may be major nematode reservoirs for sheep infection. This important conclusion raises a series of key questions. How are resistant genotypes maintained in untreated ibex hosts? Is the cost of resistance so weak that they can coexist with susceptible strains in the absence of drug treatment or does anthelminthic contamination of the pastures maintain resistant genotypes directly in wild hosts? This work also opens several interesting perspectives: For example, what additional resistant parasites may be maintained by these wildlife hosts? What role do other wild ungulate species play in the evolution of nematode communities in transhumant sheep? An expansion of this work to the larger community of wild ungulates using alpine pastures, and an evaluation of the degree to which wild species are exposed to anthelminthic drugs released by grazing livestock into the environment is now required to understand the deeper consequences of drug treatment for shaping parasite communities and their cascading impacts for wildlife conservation, and the development of efficient and sustainable management strategies for pastoral livestock. References Beaumelle et al. Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheep. bioRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.07.21.550073 Wit, J., Dilks, C.M., Andersen, E.C., 2021. Complementary Approaches with Free-living and Parasitic Nematodes to Understanding Anthelmintic Resistance. Trends Parasitol. 37, 240–250. https://doi.org/10.1016/j.pt.2020.11.008 | Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheep | Camille Beaumelle, Carole Toigo, Rodolphe Papet, Slimania Benabed, Mathieu Beurier, Lea Bordes, Anais Brignone, Nadine Curt-Grand-Gaudin, Mathieu Garel, Justine Ginot, Philippe Jacquiet, Christian Miquel, Marie-Therese Poirel, Anna Serafino, Eric ... | <p>Wild and domestic ungulates can be infected with the same species of gastrointestinal parasitic nematodes. These parasites have free-living stages in the environment that contribute to the ease of transmission among different host species. In a... |  | Ecology, Molecular biology, Parasitology | Karen D McCoy | 2023-07-25 10:36:28 | View | |
25 Aug 2021
Up and to the light: intra- and interspecific variability of photo- and geo-tactic oviposition preferences in genus TrichogrammaBurte, V., Perez, G., Ayed, F. , Groussier, G., Mailleret, L., van Oudenhove, L. and Calcagno, V. https://doi.org/10.1101/2021.03.30.437671New insights into oviposition preference of 5 Trichogramma speciesRecommended by Joël Meunier based on reviews by Kévin Tougeron and Eveline C. Verhulst based on reviews by Kévin Tougeron and Eveline C. Verhulst
Insects exhibit a great diversity of life-history traits that often vary not only between species but also between populations of the same species (Flatt and Heyland, 2011). A better understanding of the variation in these traits can be of paramount importance when it comes to species of economic and agricultural interest (Wilby and Thomas, 2002). In particular, the control of the development and expansion of agricultural pests generally requires a good understanding of the parameters that favour the reproduction of these pests and/or the reproduction of the species used to control them (Bianchi et al., 2013; Gäde and Goldsworthy, 2003). Parasitoid wasps of the genus Trichogramma are a classic example of insects involved in pest control (Smith, 1996). This genus comprises over 200 species worldwide, which have been used to control populations of a wide range of lepidopteran pests since the 1900s (Flanders, 1930; Hassan, 1993). Despite its common use, the egg-laying preference of this genus is only partially known. For example, all Trichogramma species are often thought to have positive phototaxis (or negative geotaxis) (e.g. Brower & Cline, 1984; van Atta et al., 2015), but comprehensive studies simultaneously testing this (or other) parameter among Trichogramma species and populations remain rare. This is exactly the aim of the present study (Burte et al., 2021). Using a new experimental approach based on automatic image analysis, the authors compared the photo- and geo-tactic oviposition preference among 5 Trichogramma species from 25 populations. Their results first confirm that most Trichogramma species and populations prefer light to shade, and higher to lower positions for oviposition. Interestingly, they also reveal that the levels of preference for light and gravity show inter- and intraspecific variation (probably due to local adaptation to different strata) and that both preferences tend to relax over time. Overall, this study provides important information for improving the use of Trichogramma species as biological agents. For example, it may help to establish breeding lines adapted to the microhabitat and/or growing parts of plants on which agricultural pests lay eggs most. Similarly, it suggests that the use of multiple strains with different microhabitat selection preferences could lead to better coverage of host plants, as well as a reduction in intraspecific competition in the preferred parts. Finally, this study provides a new methodology to efficiently and automatically study oviposition preferences in Trichogramma, which could be used in other insects with a particularly small size. References Bianchi, F. J. J. A., Schellhorn, N. A. and Cunningham, S. A. (2013). Habitat functionality for the ecosystem service of pest control: reproduction and feeding sites of pests and natural enemies. Agricultural and Forest Entomology, 15, 12–23. https://doi.org/10.1111/j.1461-9563.2012.00586.x Burte V., Perez G., Ayed F., Groussier G., Mailleret L, van Oudenhove L. and Calcagno V. (2021). Up and to the light: intra- and interspecific variability of photo-and geo-tactic oviposition preferences in genus Trichogramma. bioRxiv, 2021.03.30.437671, ver. 4 peer-reviewed and recommended by PCI Zoology. https://doi.org/10.1101/2021.03.30.437671 Brower, J. H. and Cline, L. D. (1984). Response of Trichogramma pretiosum and T. evanescens to Whitelight, Blacklight or NoLight Suction Traps. The Florida Entomologist, 67, 262–268. https://doi.org/10.2307/3493947 Flanders, S. E. (1930). Mass production of egg parasites of the genus Trichogramma. Hilgardia, 4, 465–501. https://doi.org/10.3733/hilg.v04n16p465 Flatt, T. and Heyland, A. (2011). Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199568765.001.0001 Gäde, G. and Goldsworthy, G. J. (2003). Insect peptide hormones: a selective review of their physiology and potential application for pest control. Pest Management Science, 59, 1063–1075. https://doi.org/10.1002/ps.755 Hassan, S. A. (1993). The mass rearing and utilization of Trichogramma to control lepidopterous pests: Achievements and outlook. Pesticide Science, 37, 387–391. https://doi.org/10.1002/ps.2780370412 Smith, S. M. (1996). Biological Control with Trichogramma : Advances, Successes, and Potential of Their Use. Annual Review of Entomology, 41, 375–406. https://doi.org/10.1146/annurev.en.41.010196.002111 van Atta, K. J., Potter, K. A. and Woods, H. A. (2015). Effects of UV-B on Environmental Preference and Egg Parasitization by Trichogramma Wasps (Hymenoptera: Trichogrammatidae). Journal of Entomological Science, 50, 318–325. https://doi.org/10.18474/JES15-09.1 Wilby, A. and Thomas, M. B. (2002). Natural enemy diversity and pest control: patterns of pest emergence with agricultural intensification. Ecology Letters, 5, 353–360. https://doi.org/10.1046/j.1461-0248.2002.00331.x | Up and to the light: intra- and interspecific variability of photo- and geo-tactic oviposition preferences in genus Trichogramma | Burte, V., Perez, G., Ayed, F. , Groussier, G., Mailleret, L., van Oudenhove, L. and Calcagno, V. | <p>Trichogramma are parasitic microwasps much used as biological control agents. The genus is known to harbor tremendous diversity, at both inter- and intra-specific levels. The successful selection of Trichogramma strains for biocontrol depends o... |  | Behavior, Biocontrol, Biodiversity, Ecology, Insecta, Parasitology, Pest management, Systematics, Terrestrial | Joël Meunier | Kévin Tougeron, Eveline C. Verhulst | 2021-04-02 16:10:28 | View |
22 Jul 2020

The open bar is closed: restructuration of a native parasitoid community following successful control of an invasive pest.David Muru, Nicolas Borowiec, Marcel Thaon, Nicolas Ris, Madalina Ionela Viciriuc, Sylvie Warot, Elodie Vercken https://doi.org/10.1101/2019.12.20.884908Raise and fall of an invasive pest and consequences for native parasitoid communitiesRecommended by Stefaniya Kamenova based on reviews by Kévin Tougeron and Miguel González Ximénez de EmbúnHost-parasitoid interactions have been the focus of extensive ecological research for decades. One the of the major reasons is the importance host-parasitoid interactions play for the biological control of crop pests. Parasitoids are the main natural regulators for a large number of economically important pest insects, and in many cases they could be the only viable crop protection strategy. Parasitoids are also integral part of complex food webs whose structure and diversity display large spatio-temporal variations [1-3]. With the increasing globalization of human activities, the generalized spread and establishment of invasive species is a major cause of disruption in local community and food web spatio-temporal dynamics. In particular, the deliberate introduction of non-native parasitoids as part of biological control programs, aiming the suppression of established, and also highly invasive crop pests, is a common practice with potentially significant, yet poorly understood effects on local food web dynamics (e.g. [4]). References [1] Eveleigh ES, McCann KS, McCarthy PC, Pollock SJ, Lucarotti CJ, Morin B, McDougall GA, Strongman DB, Huber JT, Umbanhowar J, Faria LDB (2007). Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 104, 16976-16981. doi: 10.1073/pnas.0704301104 | The open bar is closed: restructuration of a native parasitoid community following successful control of an invasive pest. | David Muru, Nicolas Borowiec, Marcel Thaon, Nicolas Ris, Madalina Ionela Viciriuc, Sylvie Warot, Elodie Vercken | <p>The rise of the Asian chestnut gall wasp *Dryocosmus kuriphilus* in France has benefited the native community of parasitoids originally associated with oak gall wasps by becoming an additional trophic subsidy and therefore perturbing population... |  | Biocontrol, Biological invasions, Ecology, Insecta | Stefaniya Kamenova | 2019-12-31 09:08:49 | View | |
01 Jul 2020

Sub-lethal insecticide exposure affects host biting efficiency of Kdr-resistant Anopheles gambiaeMalal Mamadou Diop, Fabrice Chandre, Marie Rossignol, Angelique Porciani, Mathieu Chateau, Nicolas Moiroux, Cedric Pennetier https://doi.org/10.1101/653980kdr homozygous resistant An. gambiae displayed enhanced feeding success when exposed to permethrin Insect-Treated NetsRecommended by Adrian Diaz based on reviews by Thomas Guillemaud, Niels Verhulst, Etienne Bilgo and 1 anonymous reviewerMalaria is a vector-borne parasitic disease found in 91 countries with an estimated of 228 million cases occurred worldwide during 2018. The 93% (213 million) of those cases were reported in the African Region (WHO 2019). Six species of Plasmodium parasites can produce the disease but only P. falciparum and P. vivax are the predominant species globally. More than 40 species of Anopheles mosquitoes are important malaria vectors (Asley et al. 2018). Intrinsic (genetic background, parasite susceptibility) and extrinsic (feeding host preference, host diversity and availability, mosquito abundance) factors affect the capacity of mosquitoes to vector the disease (Macdonald 1952). Malaria is prevented by chemoprophylaxis, vaccination, bite-avoidance and vector-control measures. The mainstays of vector control are long-lasting insecticide (pyrethroid) treated nets and indoor residual spraying with insecticides (Asley et al. 2018). The widespread use of pyrethroid insecticides forced the emergence of insecticide resistance in malaria vectors reducing the insecticidal effect. Mosquitoes can modify their behaviour avoiding insecticide contact and so potentially reducing vector control tools efficacy. In this sense, Diop et al. (2020) investigated whether pre-exposure to an Insecticide-Treated Net (ITN) modulates the mosquito ability to take a blood meal in Anopheles gambiae. By means of video recording experiments the authors analyzed how the feeding/bitting behaviour was affected by kdr mutation genotypes (homozygous susceptible – SS-, heterozygotes -RS- and homozygous resistant -RR-) when exposed to two different insecticides (permethrin and deltamethrin). According to the results, the blood-feeding success did not differ between the three genotypes in the absence of insecticide exposure. However, authors observed differences in the feeding duration and blood meal size. In example, RR mosquitoes spent less time taking their blood meal than RS and SS. On the other hand, RS mosquitoes took higher blood volumes than RR females. These differences can affect the mosquito fitness by decreasing/increasing the likelihood to be killed by the host defensive behavior or increase the oogenesis so enhancing fecundity. Regarding the effect of exposition to insecticides authors detected a strong relationship between kdr genotype and Knock Down (KD) phenotype when mosquitoes were exposed to Permethrin. Previously, the authors have evidenced that RR mosquitoes prefer a host protected by a permethrin-treated net rather than an untreated net and that heterozygotes RS mosquitoes have a remarkable ability to find a hole into a bet net (Diop et al. 2015, Porciani et al. 2017). With data here obtained, they demonstrated that kdr homozygous resistant An. gambiae displayed enhanced feeding success when exposed to permethrin ITN. The changes observed in the feeding/biting mosquito behaviour can affect their fitness shaping the evolution of the insecticide resistance in mosquitoes’ natural populations. Moreover, this may also alter parasite transmission dynamics by modifying vector/host interactions and so vector capacity. References World Health Organization (2019). World malaria report 2019. Geneva: World Health Organization; 2019. ISBN 978-92-4-156572-1 | Sub-lethal insecticide exposure affects host biting efficiency of Kdr-resistant Anopheles gambiae | Malal Mamadou Diop, Fabrice Chandre, Marie Rossignol, Angelique Porciani, Mathieu Chateau, Nicolas Moiroux, Cedric Pennetier | <p>The massive use of insecticide-treated nets (ITNs) has drastically changed the environment for malaria vector mosquitoes, challenging their host-seeking behaviour and biting success. Here, we investigated the effect of a brief exposure to an IT... |  | Behavior, Ecology, Evolution, Medical entomology, Pesticide resistance | Adrian Diaz | Anonymous | 2019-05-29 19:40:25 | View |
26 Aug 2022
Within and among population differences in cuticular hydrocarbons in the seabird tick Ixodes uriaeMarlène Dupraz, Chloe Leroy, Thorkell Lindberg Thórarinsson, Patrizia d’Ettorre, Karen D. McCoy https://doi.org/10.1101/2022.01.21.477272Seabird tick diversification and cuticular hydrocarbonsRecommended by Felix Sperling based on reviews by 2 anonymous reviewersTicks are notorious vectors of diseases in humans and other vertebrates. Much effort has been expended to understand tick diversity and ecology with the aim of managing their populations to alleviate the misery they bring. Further, the fundamental question of whether ticks are usually host generalists or host specialists has been debated at length and is important both for understanding the mechanisms of their diversification as well as for focusing control of ticks [1]. One elegant resolution of this question is to consider most tick species to be global generalists but local specialists [1]. This is well illustrated in a series of studies of the seabird tick, Ixodes uriae, which is comprised of host-specific races that show genetic [2], morphological [3] and host performance [4] differences associated with the seabirds they feed on. Such a pattern has clear ramifications for sympatric speciation; however, the factors that potentially act to drive these differences have remained elusive. Dupraz et al. [5] have now made intriguing and important steps toward bridging the gap between demonstrating local patterns of tick host association and understanding the physiological mechanisms that may facilitate such divergences. They collected I. uriae ticks from the nests of two seabirds – Atlantic puffins and common guillemots – on the north side of Iceland. Four populations of ticks were sampled, with one island providing both puffin ticks and guillemot ticks, to give two tick populations from each of the two seabird host species. They then washed the ticks in solvent and analyzed the dissolved cuticular hydrocarbons (CHCs) using GC mass spectrometry, revealing 22 different hydrocarbon compounds common to most of these samples. CHCs are known to be important across arthropods for a variety of functions ranging from reducing water loss to facilitating communication and recognition between individuals with species. Dupraz et al. [5] found three hydrocarbons that distinguished puffin ticks most consistently from guillemot ticks. A cross-validation test for host type also assigned 75% of the tick pools to the seabird host of origin. However, with these limited sample sizes, statistical analysis revealed no significant difference in CHC profiles between the host types, although a tendency was evident. Nonetheless, this study revealed a number of potentially diagnostic CHCs for tick host type, as well as some that may be more diagnostic of locations. This provides a fascinating and actionable foundation for further work using additional sites and host types, as well as an entry point into discerning the mechanisms at play in producing the diversity, complexity and adaptability that make ticks such medical menaces. References | Within and among population differences in cuticular hydrocarbons in the seabird tick Ixodes uriae | Marlène Dupraz, Chloe Leroy, Thorkell Lindberg Thórarinsson, Patrizia d’Ettorre, Karen D. McCoy | <p>The hydrophobic layer of the arthropod cuticle acts to maintain water balance, but can also serve to transmit chemical signals via cuticular hydrocarbons (CHC), essential mediators of arthropod behavior. CHC signatures typically vary qualitativ... |  | Acari, Biology, Ecology, Evolution | Felix Sperling | 2022-02-08 13:00:52 | View | |
09 Jul 2021
First detection of herpesvirus and mycoplasma in free-ranging Hermann tortoises (Testudo hermanni), and in potential pet vectorsJean-marie Ballouard, Xavier Bonnet, Julie Jourdan, Albert Martinez-Silvestre, Stephane Gagno, Brieuc Fertard, Sebastien Caron https://doi.org/10.1101/2021.01.22.427726Welfare threatened speciesRecommended by Peter Galbusera based on reviews by Francis Vercammen and Maria Luisa MarenzoniWildlife is increasingly threatened by drops in number of individuals and populations, and eventually by extinction. Besides loss of habitat, persecution, pet trade,… a decrease in individual health status is an important factor to consider. In this article, Ballouard et al (2021) perform a thorough analysis on the prevalence of two pathogens (herpes virus and mycoplasma) in (mainly) Western Hermann’s tortoises in south-east France. This endangered species was suspected to suffer from infections obtained through released/escaped pet tortoises. By incorporating samples of captive as well as wild tortoises, they convincingly confirm this and identify some possible ‘pet’ vectors. In February this year, a review paper on health assessments in wildlife was published (Kophamel et al 2021). Amongst others, it shows reptilia/chelonia are relatively well-represented among publications. It also contains a useful conceptual framework, in order to improve the quality of the assessments to better facilitate conservation planning. The recommended manuscript (Ballouard et al 2021) adheres to many aspects of this framework (e.g. minimum sample size, risk status, …) while others might need more (future) attention. For example, climate/environmental changes are likely to increase stress levels, which could lead to more disease symptoms. So, follow-up studies should consider conducting endocrinological investigations to estimate/monitor stress levels. Kophamel et al (2021) also stress the importance of strategic international collaboration, which may allow more testing of Eastern Hermann’s Tortoise, as these were shown to be infected by mycoplasma. The genetic health of individuals/populations shouldn’t be forgotten in health/stress assessments. As noted by Ballouard et al (2021), threatened species often have low genetic diversity which makes them more vulnerable to diseases. So, it would be interesting to link the infection data with (individual) genetic characteristics. In future research, the samples collected for this paper could fit that purpose. Finally, it is expected that this paper will contribute to the conservation management strategy of the Hermann’s tortoises. As such, it will be interesting to see how the results of the current paper will be implemented in the ‘field’. As the infections are likely caused by releases/escaped pets and as treating the wild animals is difficult, preventing them from getting infected through pets seems a priority. Awareness building among pet holders and monitoring/treating pets should be highly effective. References Ballouard J-M, Bonnet X, Jourdan J, Martinez-Silvestre A, Gagno S, Fertard B, Caron S (2021) First detection of herpesvirus and mycoplasma in free-ranging Hermann’s tortoises (Testudo hermanni), and in potential pet vectors. bioRxiv, 2021.01.22.427726, ver. 4 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2021.01.22.427726 Kophamel S, Illing B, Ariel E, Difalco M, Skerratt LF, Hamann M, Ward LC, Méndez D, Munns SL (2021), Importance of health assessments for conservation in noncaptive wildlife. Conservation Biology. https://doi.org/10.1111/cobi.13724 | First detection of herpesvirus and mycoplasma in free-ranging Hermann tortoises (Testudo hermanni), and in potential pet vectors | Jean-marie Ballouard, Xavier Bonnet, Julie Jourdan, Albert Martinez-Silvestre, Stephane Gagno, Brieuc Fertard, Sebastien Caron | <p style="text-align: justify;">Two types of pathogens cause highly contagious upper respiratory tract diseases (URTD) in Chelonians: testudinid herpesviruses (TeHV) and a mycoplasma (<em>Mycoplasma agassizii</em>). In captivity, these infections ... | Parasitology, Reptiles | Peter Galbusera | 2021-01-25 17:25:34 | View | ||
27 Apr 2023
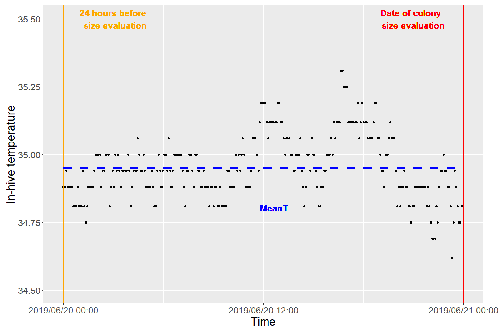
Brood thermoregulation effectivenessis positively linked to the amount of brood but not to the number of bees in honeybee coloniesUgoline Godeau, Maryline Pioz, Olivier Martin, Charlotte Rüger, Didier Crauser, Yves Le Conte, Mickael Henry, Cédric Alaux https://doi.org/10.32942/osf.io/9mwyePrecision and accuracy of honeybee thermoregulationRecommended by Michael Lattorff based on reviews by Jakob Wegener and Christopher Mayack based on reviews by Jakob Wegener and Christopher Mayack
The Western honeybee, Apis mellifera L., is one of the best-studied social insects. It shows a reproductive division of labour, cooperative brood care, and age-related polyethism. Furthermore, honeybees regulate the temperature in the hive. Although bees are invertebrates that are usually ectothermic, this is still true for individual worker bees, but the colony maintains a very narrow range of temperature, especially within the brood nest. This is quite important as the development of individuals is dependent on ambient temperature, with higher temperatures resulting in accelerated development and vice versa. In honeybees, a feedback mechanism couples developmental temperature and the foraging behaviour of the colony and the future population development (Tautz et al., 2003). Bees raised under lower temperatures are more likely to perform in-hive tasks, while bees raised under higher temperatures are better foragers. To maintain optimal levels of worker population growth and foraging rates, it is adaptive to regulate temperature to ensure optimal levels of developing brood. Moreover, this allows honeybees to decouple the internal developmental processes from ambient temperatures enhancing the ecological success of the species. In every system of thermoregulation, whether it is endothermic under the utilization of energetic resources as in mammals or the honeybee or ectothermic as in lower vertebrates and invertebrates through differential exposure to varying environmental temperature gradients, there is a need for precision (low variability) and accuracy (hitting the target temperature). However, in honeybees, the temperature is regulated by workers through muscle contraction and fanning of the wings and thus, a higher number of workers could be better at achieving precise and accurate temperature within the brood nest. Alternatively, the amount of brood could trigger responses with more brood available, a need for more precise and accurate temperature control. The authors aimed at testing these two important factors on the precision and accuracy of within-colony temperature regulation by monitoring 28 colonies equipped with temperature sensors for two years (Godeau et al., 2023). They found that the number of brood cells predicted the mean temperature (accuracy of thermoregulation). Other environmental factors had a small effect. However, the model incorporating these factors was weak in predicting the temperature as it overestimated temperatures in lower ranges and underestimated temperatures in higher ranges. In contrast, the variability of the target temperature (precision of thermoregulation) was positively affected by the external temperature, while all other factors did not show a significant effect. Again, the model was weak in predicting the data. Overall colony size measured in categories of the number of workers and the number of brood cells did not show major differences in variability of the mean temperature, but a slight positive effect for the number of bees on the mean temperature. Unfortunately, the temperature was a poor predictor of colony size. The latter is important as the remote control of beehives using Internet of Things (IoT) technologies get more and more incorporated into beekeeping management. These IoT technologies and their success are dependent on good proxies for the control of the status of the colony. Amongst the factors to monitor, the colony size (number of bees and/or amount of brood) is extremely important, but temperature measurements alone will not allow us to predict colony sizes. Nevertheless, this study showed clearly that the number of brood cells is a crucial factor for the accuracy of thermoregulation in the beehive, while ambient temperature affects the precision of thermoregulation. In the view of climate change, the latter factor seems to be important, as more extreme environmental conditions in the future call for measures of mitigation to ensure the proper functioning of the bee colony, including the maintenance of homeostatic conditions inside of the nest to ensure the delivery of the ecosystem service of pollination. REFERENCES Godeau U, Pioz M, Martin O, Rüger C, Crauser D, Le Conte Y, Henry M, Alaux C (2023) Brood thermoregulation effectiveness is positively linked to the amount of brood but not to the number of bees in honeybee colonies. EcoEvoRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.32942/osf.io/9mwye Tautz J, Maier S, Claudia Groh C, Wolfgang Rössler W, Brockmann A (2003) Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. PNAS 100: 7343–7347. https://doi.org/10.1073/pnas.1232346100 | Brood thermoregulation effectivenessis positively linked to the amount of brood but not to the number of bees in honeybee colonies | Ugoline Godeau, Maryline Pioz, Olivier Martin, Charlotte Rüger, Didier Crauser, Yves Le Conte, Mickael Henry, Cédric Alaux | <p style="text-align: justify;">To ensure the optimal development of brood, a honeybee colony needs to regulate its temperature within a certain range of values (thermoregulation), regardless of environmental changes in biotic and abiotic factors.... |  | Biology, Conservation biology, Demography/population dynamics, Ecology, Insecta | Michael Lattorff | Mauricio Daniel Beranek | 2022-07-06 09:20:10 | View |
08 Mar 2024
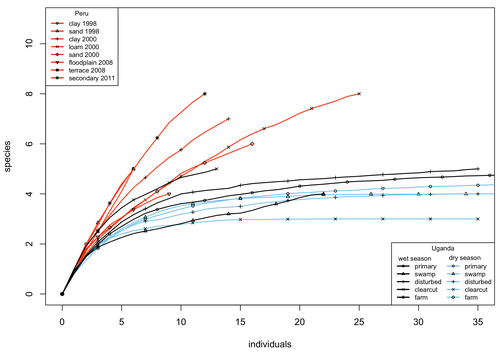
A comparison of the parasitoid wasp species richness of tropical forest sites in Peru and Uganda – subfamily Rhyssinae (Hymenoptera: Ichneumonidae)Tapani Hopkins, Hanna Tuomisto, Isrrael C. Gómez, Ilari E. Sääksjärvi https://doi.org/10.1101/2023.08.23.554460Two sides of tropical richness, parasitoid wasps collected by Malaise traps in tropical rainforests of South America and AfricaRecommended by Giovanny Fagua based on reviews by Mabel Alvarado, Filippo Di Giovanni and 2 anonymous reviewersInsect species richness and diversity comparisons between samples of the tropics around the world are rare, especially in taxa composed mainly of cryptic species as parasitoid wasps. The article by Hopkins et al. (2024) compares samples of parasitoid wasps of the subfamily Rhyssinae (Hymenoptera: Ichneumonidae) collected by Malaise traps in tropical rainforests of Perú and Uganda. The samples presented several differences in the time of collecting, covertures, and the sampling number; however, they used the same kind of traps, and the taxonomic process for species delimitation was made for the same team of ichneumonid experts, using equivalent characters. Publications about this kind of comparative study are difficult to find because cooperative projects on insect richness and diversity from South American and African continents are not frequent. In this sense, this study presented a valuable contrast that shows interesting results about the higher richness and lower abundance of the biota of the American tropics, even with a small sample, in comparison with the biota of the African tropics. The results are supported mainly by the rarefaction curves shown. This pattern of higher species richness and lower specimen abundance, observed in other American tropical taxa such as trees, birds, or butterflies, is observed too in these parasitoid wasps, increasing the body of information that could support the extension of the pattern to the entire biota of the American tropics. The authors recognize the study's limitations, which include strong differences in the size of the forest coverture between places. However, these differences and others are enough described and discussed. This work is useful because it increases the information about the diversity patterns of the tropics around the world and because study a taxon mainly composed of cryptic species, with a small amount of information in tropical regions. References Hopkins T., Tuomisto H., Gómez I.C., Sääksjärvi I. E. 2024. A comparison of the parasitoid wasp species richness of tropical forest sites in Peru and Uganda – subfamily Rhyssinae (Hymenoptera: Ichneumonidae). bioRxiv, ver. 2 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.08.23.554460 | A comparison of the parasitoid wasp species richness of tropical forest sites in Peru and Uganda – subfamily Rhyssinae (Hymenoptera: Ichneumonidae) | Tapani Hopkins, Hanna Tuomisto, Isrrael C. Gómez, Ilari E. Sääksjärvi | <p style="text-align: justify;">The global distribution of parasitoid wasp species richness is poorly known. Past attempts to compare data from different sites have been hampered by small sample sizes and lack of standardisation. During the past d... |  | Biodiversity, Biogeography, Insecta | Giovanny Fagua | 2023-08-24 18:30:26 | View | |
19 Jul 2024

Museomics of Carabus giant ground beetles shows an Oligocene origin and in situ Alpine diversificationMarie T. PAULI, Jeremy GAUTHIER, Marjorie LABEDAN, Mickael BLANC, Julia BILAT, Emmanuel F.A. TOUSSAINT https://doi.org/10.1101/2024.03.21.586057Natural history collections continue to inform ground beetle genetics.Recommended by Felix Sperling based on reviews by Michael Caterino, Julian Dupuis and 1 anonymous reviewerSome of the biodiversity of our planet now exists only in museums, due to continuing habitat destruction and climate change. With more than 380 million entomological specimens already preserved in museums (Johnson and Owens 2023), there is much work left to document what we already have. Fortunately, new advances in DNA sequencing have given us the opportunity to get enormous amounts of information from dried specimens on pins. Johnson KR, Owens, (IFP. 2023) A global approach for natural history museum collections. Science 379,1192-1194(2023). https://doi.org/10.1126/science.adf6434 Pauli MT, Gauthier J, Labédan M, Blanc M, Bilat J, Toussaint EFA (2024) Museomics of Carabus giant ground beetles shows an Oligocene origin and in situ alpine diversification. bioRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2024.03.21.586057 Schmid, S., Genevest, R., Gobet, E., Suchan, T., Sperisen, C., Tinner, W. and Alvarez, N. (2017), HyRAD-X, a versatile method combining exome capture and RAD sequencing to extract genomic information from ancient DNA. Methods Ecol Evol, 8: 1374-1388. https://doi.org/10.1111/2041-210X.12785 Toussaint EFA, Gauthier J, Bilat J, Gillett CPDT, Gough HM, Lundkvist H, Blanc M, Muñoz-Ramírez CP, Alvarez N (2021) HyRAD-X Exome Capture Museomics Unravels Giant Ground Beetle Evolution, Genome Biology and Evolution, Volume 13, Issue 7, evab112, https://doi.org/10.1093/gbe/evab112 | Museomics of *Carabus* giant ground beetles shows an Oligocene origin and *in situ* Alpine diversification | Marie T. PAULI, Jeremy GAUTHIER, Marjorie LABEDAN, Mickael BLANC, Julia BILAT, Emmanuel F.A. TOUSSAINT | <p style="text-align: justify;">The development of museomics represents a major paradigm shift in the use of natural history collection specimens for systematics and evolutionary biology. New approaches in this field allow the sequencing of hundre... |  | Insecta, Phylogeny, Systematics | Felix Sperling | 2024-03-27 15:30:31 | View | |
09 Feb 2023

A novel nematode species from the Siberian permafrost shares adaptive mechanisms for cryptobiotic survival with C. elegans dauer larvaAnastasia Shatilovich, Vamshidhar R. Gade, Martin Pippel, Tarja T. Hoffmeyer, Alexei V. Tchesunov, Lewis Stevens, Sylke Winkler, Graham M. Hughes, Sofia Traikov, Michael Hiller, Elizaveta Rivkina, Philipp H. Schiffer, Eugene W Myers, Teymuras V. Kurzchalia https://doi.org/10.1101/2022.01.28.478251A novel nematode species from the Siberian permafrost shares adaptive mechanisms for cryptobiotic survival with C. elegans dauer larvaRecommended by Isa Schon based on reviews by 3 anonymous reviewersThis article [1] investigated two nematode genera, Panagrolaimus and Plectus, from the Siberian permafrost to unravel the adaptations allowing them to survive cryptobiosis; radio carbon dating showed that the individuals of Panagrolaimus had been in cryobiosis in Siberia for as long as 46,000 years! I was impressed by the multidisciplinary approach of this study, including morphological as well as phylogenetic and -genomic analyses to describe a new species. In triploids as some of the species studied here, it is quite challenging to assemble a novel genome. The authors furthermore not only managed to successfully reanimate the Siberian specimens but could also expose them to repeated freezing and desiccation in the lab, not an easy task. This study reports some amazing discoveries - comparing the molecular toolkits between C. elegans and Panagrolaimus and Plectus revealed that several components were orthologues. Likewise, some of the biochemical mechanisms for surviving freezing in the lab turned out to be similar for C. elegans and the Siberian nematodes. This study thus provides strong evidence that nematodes developed specific mechanisms allowing them to stay in cryobiosis over very long times. A surprising additional experimental result concerns the well-studied C. elegans - dauer larvae of this species can stay viable much longer after periods of animated suspension than previously thought. I highly recommend this article as it is an important contribution to the fields of evolution and molecular biology. This study greatly advanced our understanding of how nematodes could have adapted to cryobiosis. The applied techniques could also be useful for studying similar research questions in other organisms. Reference [1] Shatilovich A, Gade VR, Pippel M, Hoffmeyer TT, Tchesunov AV, Stevens L, Winkler S, Hughes GM, Traikov S, Hiller M, Rivkina E, Schiffer PH, Myers EW, Kurzchalia TV (2023) A novel nematode species from the Siberian permafrost shares adaptive mechanisms for cryptobiotic survival with C. elegans dauer larva. bioRxiv, 2022.01.28.478251, ver. 6 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2022.01.28.478251 | A novel nematode species from the Siberian permafrost shares adaptive mechanisms for cryptobiotic survival with C. elegans dauer larva | Anastasia Shatilovich, Vamshidhar R. Gade, Martin Pippel, Tarja T. Hoffmeyer, Alexei V. Tchesunov, Lewis Stevens, Sylke Winkler, Graham M. Hughes, Sofia Traikov, Michael Hiller, Elizaveta Rivkina, Philipp H. Schiffer, Eugene W Myers, Teymuras V. K... | <p style="text-align: justify;">Some organisms in nature have developed the ability to enter a state of suspended metabolism called cryptobiosis1 when environmental conditions are unfavorable. This state-transition requires the execution of comple... |  | Ecology, Evolution, Genetics/Genomics | Isa Schon | 2022-05-20 14:32:02 | View |
MANAGING BOARD
Dominique Adriaens
Ellen Decaestecker
Benoit Facon
Isabelle Schon
Emmanuel Toussaint
Bertanne Visser










