Latest recommendations
| Id | Title * | Authors * | Abstract * | Picture * | Thematic fields * | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
13 Dec 2024
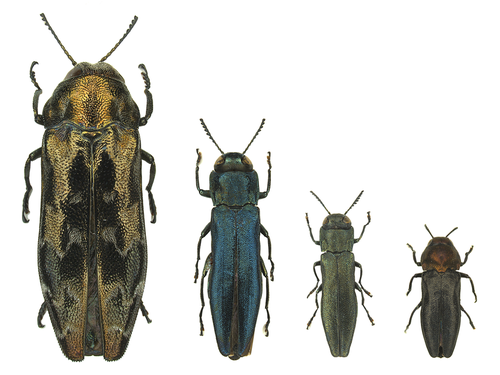
Intra- and interspecific variations in flight performance of oak-associated Agrilinae (Coleoptera: Buprestidae) using computerised flight millsElodie Le Souchu, Aurélien Sallé, Stéphanie Bankhead-Dronnet, Mathieu Laparie, Daniel Sauvard https://doi.org/10.1101/2024.07.01.601558A comparative study of flight performance and the factors affecting the flight behaviour of oak-associated Agrilinae (Coleoptera: Buprestidae)Recommended by Pedro Abellan based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
Some insects are known to be phytosanitary threats on a wide diversity of plants and can have important economic and ecological impacts in their native area. This is the case of some species within the jewel beetle subfamily Agrilinae (Coleoptera: Buprestidae), which are associated with broadleaf forest declines and diebacks (Jendek & Poláková, 2014). These thermophilous borers are expected to be favoured by climate change and the global deterioration of forest health, and ultimately expand their range and damage. Active flight plays a crucial role in the life strategies of most insects, facilitating essential activities such as mate searching, locating trophic resources, finding favorable environmental conditions, and dispersing to or colonizing new geographic areas (Dudley, 2002). Studying flight capacities provides valuable insights into the ecology of these species and helps estimate their ability to spread within new environments. Assessing the flight capacities of pest and alien species is therefore critical for evaluating their dispersal potential and for designing effective monitoring and control strategies. The study by Le Souchu et al. (2024) aimed to assess intra- and interspecific variability in active flight of several Agrilinae species and to evaluate the effects of sex and mass on this variability. Using computerised flight mills, they assessed the flight performance of twelve species, most of them associated with oaks. A key feature of the study is the extensive dataset used, which reveals significant variability in flight distance and capacity among species and individuals. Body mass positively influenced flight capacity in some species, while no sexual dimorphism was observed. The findings suggest a generally low average dispersal propensity within these species and highlight the critical role of rare, exceptional individuals in driving colonization and spread patterns at both population and species levels. Overall, the study provides a valuable comparative analysis of flight behavior and performance in several Agrilinae species associated with oak forests. Because flight behaviour and performance of these insects are poorly known despite their critical role in dispersal inside and outside native ranges and their relevance for management purposes, this study contributes to filling this gap. From a broader perspective, the findings revealed several common traits among the studied species and provide insights into the influence of different factors on flight activity. References Dudley, R. (2002) The biomechanics of insect flight: form, function, evolution. Princeton University Press, Princeton, N.J. https://doi.org/10.1515/9780691186344 Jendek, E., Poláková, J. (2014) Host Plants of World Agrilus (Coleoptera, Buprestidae). Springer International Publishing, Cham. https://doi.org/10.1007/978-3-319-08410-7 Le Souchu, E., Sallé, A., Bankhead-Dronnet, S., Laparie, M., Sauvard, S. (2024) Intra- and interspecific variations in flight performance of oak-associated Agrilinae (Coleoptera: Buprestidae) using computerised flight mills . bioRxiv, ver.2 peer-reviewed and recommended by PCI Zoology https://doi.org/10.1101/2024.07.01.601558
| Intra- and interspecific variations in flight performance of oak-associated Agrilinae (Coleoptera: Buprestidae) using computerised flight mills | Elodie Le Souchu, Aurélien Sallé, Stéphanie Bankhead-Dronnet, Mathieu Laparie, Daniel Sauvard | <p style="text-align: justify;">Several Agrilinae species (Coleoptera: Buprestidae) are secondary pests of broadleaf forests, and some of them are also major invasive pests. These thermophilous borers are expected to be favoured by climate change ... |  | Behavior, Biology, Ecology, Insecta | Pedro Abellan | 2024-07-05 22:50:58 | View | |
14 Oct 2024

Negative impact of mild arid conditions on a rodent revealed using a physiological approach in naturaHamilcar S. Keilani, Nico L. Avenant, Pierre Caminade, Neville Pillay, Guila Ganem https://doi.org/10.1101/2024.03.11.583554Physiological Adaptations to Arid Conditions in South African Rodents: A Comparative Study of Rhabdomys SpeciesRecommended by Vincent Foray based on reviews by 2 anonymous reviewersUnderstanding how organisms are affected by environmental variations is a central question in ecophysiology and evolutionary ecology, particularly in the context of global changes(Fuller et al., 2016). Environmental variations challenge organisms' ability to maintain homeostasis leading to divergent adaptations between habitat specialists and generalists (Kawecki and Ebert, 2004). The article by (Keilani et al.) (2024) presents an original contribution to this field by focusing on the response to dry conditions in two rodent species from semi-arid regions of South Africa. The two species, Rhabdomys bechuanae and R.dilectus dilectus, have different environmental niches : R.dilectus dilectus occurring in mesic habitats while R. bechuanae is found in semi-arid and arid habitats. Previous studies highlighted morphological and behavioral adaptations to arid conditions in R. bechuanae (Dufour et al., 2019), the current study focuses on the physiological responses of the two species to seasonal dry conditions. By analyzing body condition, markers of kidney and liver functions, and habitat characteristics the authors aim to understand how aridity impacts parapatric populations of the two species. They hypothesize that i) the aridity of the habitat tend to increase during the dry season, ii) both species can adjust their physiology to dry conditions thanks to phenotypic plasticity, and iii) R. bechuanae, having evolved in arid environments, will cope better with dry conditions than R. d. dilectus. References Dufour, C.M.S., Pillay, N., Avenant, N., Watson, J., Loire, E., and Ganem, G. (2019) Habitat characteristics and species interference influence space use and nest-site occupancy: implications for social variation in two sister species. Oikos128: 503-516. Hamilcar S. Keilani, Nico L. Avenant, Pierre Caminade, Neville Pillay, Guila Ganem (2024) Negative impact of mild arid conditions on a rodent revealed using a physiological approach in natura. bioRxiv, ver.9 peer-reviewed and recommended by PCI Zoology | Negative impact of mild arid conditions on a rodent revealed using a physiological approach in natura | Hamilcar S. Keilani, Nico L. Avenant, Pierre Caminade, Neville Pillay, Guila Ganem | <p>1. Understanding how organisms respond to seasonal variations in their environment can be a window to their potential adaptability, a classical problem in evolutionary ecology. In the context of climate change, inducing increased aridity and di... |  | Ecology, Evolution, Physiology | Vincent Foray | 2024-05-02 18:38:29 | View | |
19 Aug 2024
Dose, temperature and formulation shape Metarhizium anisopliae virulence against the oriental fruit fly: lessons for improving on-target control strategiesAnais Chailleux, Oumou N. Coulibaly, Babacar Diouf, Samba Diop, Ahmad Sohel, Thierry Brevault https://doi.org/10.1101/2023.12.14.571642Optimizing fungal pathogen strategies for oriental fruit fly controlRecommended by Kévin Tougeron based on reviews by François Verheggen and Papa Djibril Faye based on reviews by François Verheggen and Papa Djibril Faye
Using entomopathogenic fungi for biological control is an effective method for controlling certain crop pests, with the perspective of reducing the use of chemical pesticides. Yet, the efficiency of pathogenic fungi is dependent upon many factors that need to be evaluated to improve biological control potential in the fields (Lacey, 2001). The article by Chailleux et al. (2024) presents an exciting contribution to the field of biological pest control, specifically focusing on using entomopathogenic fungi to manage the oriental fruit fly, Bactrocera dorsalis. This fly, a member of the Tephritidae family, is a major threat to orchards in Asia, the Pacific and Africa, as it attacks fruit and causes considerable damage, in addition to having a relatively rapid biological invasion dynamic (Clarke et al. 2005). The objective of the Chailleux et al. (2024) study was to evaluate the virulence of Metarhizium anisopliae spores (strain Met69) on B. dorsalis adult flies according to various conditions: the inoculation dose and spore load, the formulation (adjuvant) and temperature conditions. The focus on host specificity and on-target applications was conducted to ensure minimal impact on non-target organisms, which is crucial for sustainable agriculture. The main challenge in this system was to achieve high strain virulence to kill wild individuals with a low number of spores—therefore limiting impact on non-target species such as natural enemies—but with a sufficient incubation period to allow transmission from mass-reared insects to wild conspecifics (Leite et al. 2022). A comparison of different inoculation methods is also provided and is interesting from a methodological point of view for future studies or even large-scale applications. Using a well-designed experimental setup, the authors show that high pathogenicity (measured by LD50) is achievable even at low spore doses and independently of the fly's sex. Lethal action speed was, however, dependent on the dose. Regarding temperature, the authors demonstrated that mycelium growth was affected by the mean temperature but, most importantly, by daily fluctuation regimes; night and day temperature alternation allowed faster growth than constant temperature. These notions of thermal fluctuations are still under-researched in terms of their modulating role in biological control yet seem central to understanding them, as the authors demonstrate here. The correlation between increased virulence and specific abiotic factors, such as temperature, offers valuable additional insights into the bioecology of the insect host and the fungal pathogen. Chailleux et al. finally point out the need for careful selection of adjuvants in formulations and pay attention to interactions with the abiotic environment to avoid compromising the effectiveness of biological control agents. Indeed, the survival rate of inoculated flies increased in the presence of the corn starch adjuvant, but this effect decreased with temperature. As corn starch unexpectedly delayed mortality, the authors suggest a potential for enhancing conspecific transmission From a broader perspective, the study emphasizes the importance of standardizing virulence evaluation to optimize biological control strategies like auto-dissemination or vectoring with sterile males, particularly in field conditions. The study contributions are timely and essential for advancing sustainable pest management strategies and improving inoculation methods. The findings underscore the need for field trials to refine these strategies, particularly in Africa, where climatic factors may affect pathogen efficacy and fly behavior. I recommend publishing this article in a referenced journal like the Peer Community Journal. References Chailleux, A. Coulibaly, ON, Diouf B, Diop S, Sohel A, Brevault T (2023) Dose, temperature and formulation shape Metarhizium anisopliae virulence against the oriental fruit fly: lessons for improving on-target control strategies. bioRxiv, ver.2 peer-reviewed and recommended by PCI Zoology https://doi.org/10.1101/2023.12.14.571642 Clarke, A. R. et al. (2005). Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol., 50, 293-319. https://doi.org/10.1146/annurev.ento.50.071803.130428 Lacey, L. A. (2001). Formulation of microbial biopesticides: beneficial microorganisms, nematodes and seed treatments. J Invertebr Pathol, 77, 147. https://doi.org/10.1006/jipa.2000.5005 Leite, M. O. et al. (2022). Laboratory risk assessment of three entomopathogenic fungi used for pest control toward social bee pollinators. Microorganisms, 10, 1800. https://doi.org/10.3390/microorganisms10091800 | Dose, temperature and formulation shape Metarhizium anisopliae virulence against the oriental fruit fly: lessons for improving on-target control strategies | Anais Chailleux, Oumou N. Coulibaly, Babacar Diouf, Samba Diop, Ahmad Sohel, Thierry Brevault | <p>Entomopathogenic fungi are a promising tool for the biological control of crop pests provided low or no impact on non-target organisms. Selection for host specificity as well as on-target applications open new avenues for more sustainable stra... |  | Biocontrol, Insecta, Pest management | Kévin Tougeron | 2023-12-18 11:59:30 | View | |
31 Jul 2024
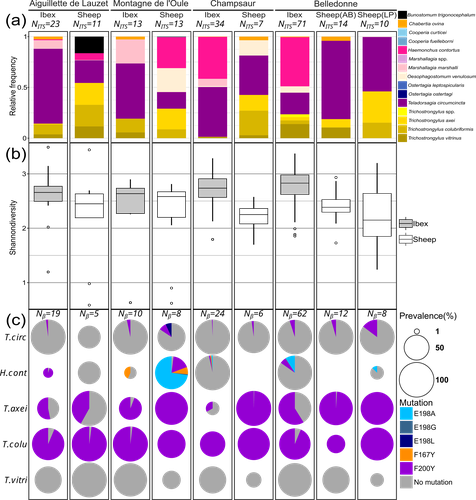
Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheepCamille Beaumelle, Carole Toigo, Rodolphe Papet, Slimania Benabed, Mathieu Beurier, Lea Bordes, Anais Brignone, Nadine Curt-Grand-Gaudin, Mathieu Garel, Justine Ginot, Philippe Jacquiet, Christian Miquel, Marie-Therese Poirel, Anna Serafino, Eric Vannard, Gilles Bourgoin, Glenn Yannic https://doi.org/10.1101/2023.07.21.550073What gets left behind? Shared nematode communities at the wildlife-livestock interface.Recommended by Karen D McCoy based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
Gastrointestinal nematodes represent a major problem for livestock production across the globe, one that has intensified with the rapid and repeated evolution of multi-drug resistance (Wit et al., 2021). Understanding parasite exposure and how resistance is maintained over time are therefore of key importance for defining efficient management strategies. To date, the role wildlife play in these dynamics has been poorly studied. The work of Beaumelle et al. examine this essential question by studying the transmission dynamics of nematodes at the environmental interface between transhumant sheep and wild ungulates, more specifically with ibex (Capra ibex) that allochronically share alpine pastures when sheep are brought to graze in summer. By collecting fresh fecal material from both species and using a metabarcoding approach based on ITS-2 sequences, the authors characterise the nemabiome in each ungulate species and demonstrate that the two host species share a large portion of their parasite diversity. More importantly, by focusing on a gene (β-tubulin isotype 1) associated with resistance to a commonly used anthelmintic drug (benzimidazole), they demonstrate that both species carry resistant nematode strains, but that the diversity of strains, and particularly susceptible strains, is much higher in ibex. A key feature of the sampling design is that fecal material from both species was collected before seasonal transmission between the ungulate species could occur. Therefore, their results demonstrate that ibex are able to maintain resistant strains over long periods of time and therefore may be major nematode reservoirs for sheep infection. This important conclusion raises a series of key questions. How are resistant genotypes maintained in untreated ibex hosts? Is the cost of resistance so weak that they can coexist with susceptible strains in the absence of drug treatment or does anthelminthic contamination of the pastures maintain resistant genotypes directly in wild hosts? This work also opens several interesting perspectives: For example, what additional resistant parasites may be maintained by these wildlife hosts? What role do other wild ungulate species play in the evolution of nematode communities in transhumant sheep? An expansion of this work to the larger community of wild ungulates using alpine pastures, and an evaluation of the degree to which wild species are exposed to anthelminthic drugs released by grazing livestock into the environment is now required to understand the deeper consequences of drug treatment for shaping parasite communities and their cascading impacts for wildlife conservation, and the development of efficient and sustainable management strategies for pastoral livestock. References Beaumelle et al. Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheep. bioRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.07.21.550073 Wit, J., Dilks, C.M., Andersen, E.C., 2021. Complementary Approaches with Free-living and Parasitic Nematodes to Understanding Anthelmintic Resistance. Trends Parasitol. 37, 240–250. https://doi.org/10.1016/j.pt.2020.11.008 | Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheep | Camille Beaumelle, Carole Toigo, Rodolphe Papet, Slimania Benabed, Mathieu Beurier, Lea Bordes, Anais Brignone, Nadine Curt-Grand-Gaudin, Mathieu Garel, Justine Ginot, Philippe Jacquiet, Christian Miquel, Marie-Therese Poirel, Anna Serafino, Eric ... | <p>Wild and domestic ungulates can be infected with the same species of gastrointestinal parasitic nematodes. These parasites have free-living stages in the environment that contribute to the ease of transmission among different host species. In a... |  | Ecology, Molecular biology, Parasitology | Karen D McCoy | 2023-07-25 10:36:28 | View | |
19 Jul 2024

Museomics of Carabus giant ground beetles shows an Oligocene origin and in situ Alpine diversificationMarie T. PAULI, Jeremy GAUTHIER, Marjorie LABEDAN, Mickael BLANC, Julia BILAT, Emmanuel F.A. TOUSSAINT https://doi.org/10.1101/2024.03.21.586057Natural history collections continue to inform ground beetle genetics.Recommended by Felix Sperling based on reviews by Michael Caterino, Julian Dupuis and 1 anonymous reviewerSome of the biodiversity of our planet now exists only in museums, due to continuing habitat destruction and climate change. With more than 380 million entomological specimens already preserved in museums (Johnson and Owens 2023), there is much work left to document what we already have. Fortunately, new advances in DNA sequencing have given us the opportunity to get enormous amounts of information from dried specimens on pins. Johnson KR, Owens, (IFP. 2023) A global approach for natural history museum collections. Science 379,1192-1194(2023). https://doi.org/10.1126/science.adf6434 Pauli MT, Gauthier J, Labédan M, Blanc M, Bilat J, Toussaint EFA (2024) Museomics of Carabus giant ground beetles shows an Oligocene origin and in situ alpine diversification. bioRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2024.03.21.586057 Schmid, S., Genevest, R., Gobet, E., Suchan, T., Sperisen, C., Tinner, W. and Alvarez, N. (2017), HyRAD-X, a versatile method combining exome capture and RAD sequencing to extract genomic information from ancient DNA. Methods Ecol Evol, 8: 1374-1388. https://doi.org/10.1111/2041-210X.12785 Toussaint EFA, Gauthier J, Bilat J, Gillett CPDT, Gough HM, Lundkvist H, Blanc M, Muñoz-Ramírez CP, Alvarez N (2021) HyRAD-X Exome Capture Museomics Unravels Giant Ground Beetle Evolution, Genome Biology and Evolution, Volume 13, Issue 7, evab112, https://doi.org/10.1093/gbe/evab112 | Museomics of *Carabus* giant ground beetles shows an Oligocene origin and *in situ* Alpine diversification | Marie T. PAULI, Jeremy GAUTHIER, Marjorie LABEDAN, Mickael BLANC, Julia BILAT, Emmanuel F.A. TOUSSAINT | <p style="text-align: justify;">The development of museomics represents a major paradigm shift in the use of natural history collection specimens for systematics and evolutionary biology. New approaches in this field allow the sequencing of hundre... |  | Insecta, Phylogeny, Systematics | Felix Sperling | 2024-03-27 15:30:31 | View | |
07 Jun 2024

Relationship between weapon size and six key behavioural and physiological traits in males of the European earwigSamantha E.M. Blackwell, Laura Pasquier, Simon Dupont, Séverine Devers, Charlotte Lécureuil, *Joël Meunier https://doi.org/10.1101/2024.03.20.585871The unreliable signal: No correlation between forceps length and male quality in European earwigsRecommended by Olivier Roux based on reviews by Luna Grey and 2 anonymous reviewers based on reviews by Luna Grey and 2 anonymous reviewers
In animals, male weapons such as antlers, horns, spurs, fangs, and tusks typically provide advantages in male contests and increase access to females, thereby enhancing reproductive success. However, such large and extravagant morphological structures are expected to come at a cost, potentially imposing trade-offs with life history traits, physiological functions, or certain behaviors (Emlen, 2001; Emlen, 2008). These costs should be manageable only by males in the best condition. The present study by Blackwell et al. (2024) examines this assumption through a comprehensive study on the European earwig, where males possess forceps-like cerci that vary widely in size within populations. In the European earwig (Forficula auricularia), male forceps are used in male-male contests as weapons to deter competitors prior to mating (Styrsky & Rhein, 1999) or to interrupt mating pairs by non-copulating males (Forslund, 2000; Walker & Fell, 2001). Despite providing benefits in terms of mating success (Eberhard & Gutierrez, 1991; Tomkins & Brown, 2004), it remains unknown whether long or short forceps are associated with other important life-history traits. In this laboratory study, Blackwell et al. (2024) investigated two European earwig populations, each divided into two subpopulations: one with the shortest forceps and one with the longest forceps. They examined the potential costs of long forceps on six different traits: one reproductive trait (sperm storage); three non-reproductive behavioral traits such as locomotor performance (involved in search for resources), fleeing reaction face to a risk (long forceps are supposed to be correlated with boldness), and aggregation behavior (European earwigs are facultative group-living organisms); and survival (when deprived of food and subsequently when exposed to an entomopathogenic fungus). As males in the best condition are supposed to be those that can afford to develop large forceps, Blackwell et al. (2024) predicted that males with long forceps would perform better than those with short forceps across the investigated traits. However, their predictions were not validated, as no correlation between weapon size and male quality was detected in either population. Although the sample size is sometimes limited, the consistency of these results across different populations adds robustness to their conclusions. By demonstrating that forceps length in the European earwig does not reliably indicate male quality, this paper challenges existing theories and highlights the complexity of evolutionary processes shaping morphological traits. Furthermore, the study raises important questions about the evolutionary mechanisms maintaining weapon size diversity, providing a fresh perspective that could stimulate further research and debate in the field, notably the search for other traits where costs might be incurred. References Blackwell, S.E.M., Pasquier, L., Dupont, S., Devers, S., Lécureuil, C. & Meunier, J. (2024). Relationship between weapon size and six key behavioural and physiological traits in males of the European earwig. bioRxiv, ver. 3 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2024.03.20.585871 Eberhard, W.G., & Gutierrez, E.E. (1991). Male dimorphisms in beetles and earwigs and the question of developmental constraints. Evolution, 45(1), 18–28. https://doi.org/10.2307/2409478 Emlen, D.J. (2001). Costs and the diversification of exaggerated animal structures. Science, 291(5508), 1534–1536. https://doi.org/10.1126/science.1056607 Emlen, D.J. (2008). The evolution of animal weapons. Annual Review of Ecology, Evolution, and Systematics, 39(1), 387–413. https://doi.org/10.1146/annurev.ecolsys.39.110707.173502 Forslund, P. (2000). Male-male competition and large size mating advantage in European earwigs, Forficula auricularia. Animal Behaviour, 59(4), 753–762. https://doi.org/10.1006/anbe.1999.1359 Styrsky, J.D., & Rhein, S.V. (1999). Forceps size does not determine fighting success in European earwigs. Journal of Insect Behavior, 12(4), 475–482. https://doi.org/10.1023/A:1020962606724 Tomkins, J.L., & Brown, G.S. (2004). Population density drives the local evolution of a threshold dimorphism. Nature, 431, 1099–1103. https://doi.org/10.1038/nature02936.1. Walker, K.A., & Fell, R.D. (2001). Courtship roles of male and female European earwigs, Forficula auricularia L. (Dermaptera: Forficulidae), and sexual use of forceps. Journal of Insect Behavior, 14(1), 1–17. https://doi.org/10.1023/A:1007843227591 | Relationship between weapon size and six key behavioural and physiological traits in males of the European earwig | Samantha E.M. Blackwell, Laura Pasquier, Simon Dupont, Séverine Devers, Charlotte Lécureuil, *Joël Meunier | <p style="text-align: justify;">In many animals, male weapons are large and extravagant morphological structures that typically enhance fighting ability and reproductive success. It is generally assumed that growing and carrying large weapons is c... |  | Behavior, Evolution, Insecta, Invertebrates, Life histories, Morphology | Olivier Roux | 2024-03-26 08:56:27 | View | |
08 Mar 2024
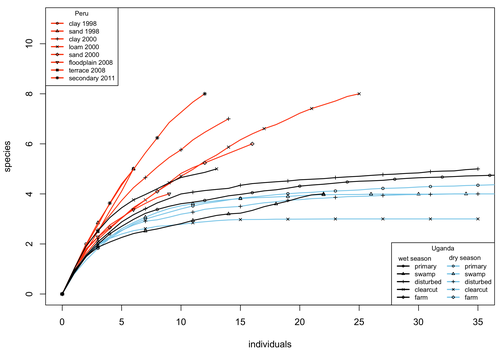
A comparison of the parasitoid wasp species richness of tropical forest sites in Peru and Uganda – subfamily Rhyssinae (Hymenoptera: Ichneumonidae)Tapani Hopkins, Hanna Tuomisto, Isrrael C. Gómez, Ilari E. Sääksjärvi https://doi.org/10.1101/2023.08.23.554460Two sides of tropical richness, parasitoid wasps collected by Malaise traps in tropical rainforests of South America and AfricaRecommended by Giovanny Fagua based on reviews by Mabel Alvarado, Filippo Di Giovanni and 2 anonymous reviewersInsect species richness and diversity comparisons between samples of the tropics around the world are rare, especially in taxa composed mainly of cryptic species as parasitoid wasps. The article by Hopkins et al. (2024) compares samples of parasitoid wasps of the subfamily Rhyssinae (Hymenoptera: Ichneumonidae) collected by Malaise traps in tropical rainforests of Perú and Uganda. The samples presented several differences in the time of collecting, covertures, and the sampling number; however, they used the same kind of traps, and the taxonomic process for species delimitation was made for the same team of ichneumonid experts, using equivalent characters. Publications about this kind of comparative study are difficult to find because cooperative projects on insect richness and diversity from South American and African continents are not frequent. In this sense, this study presented a valuable contrast that shows interesting results about the higher richness and lower abundance of the biota of the American tropics, even with a small sample, in comparison with the biota of the African tropics. The results are supported mainly by the rarefaction curves shown. This pattern of higher species richness and lower specimen abundance, observed in other American tropical taxa such as trees, birds, or butterflies, is observed too in these parasitoid wasps, increasing the body of information that could support the extension of the pattern to the entire biota of the American tropics. The authors recognize the study's limitations, which include strong differences in the size of the forest coverture between places. However, these differences and others are enough described and discussed. This work is useful because it increases the information about the diversity patterns of the tropics around the world and because study a taxon mainly composed of cryptic species, with a small amount of information in tropical regions. References Hopkins T., Tuomisto H., Gómez I.C., Sääksjärvi I. E. 2024. A comparison of the parasitoid wasp species richness of tropical forest sites in Peru and Uganda – subfamily Rhyssinae (Hymenoptera: Ichneumonidae). bioRxiv, ver. 2 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.08.23.554460 | A comparison of the parasitoid wasp species richness of tropical forest sites in Peru and Uganda – subfamily Rhyssinae (Hymenoptera: Ichneumonidae) | Tapani Hopkins, Hanna Tuomisto, Isrrael C. Gómez, Ilari E. Sääksjärvi | <p style="text-align: justify;">The global distribution of parasitoid wasp species richness is poorly known. Past attempts to compare data from different sites have been hampered by small sample sizes and lack of standardisation. During the past d... |  | Biodiversity, Biogeography, Insecta | Giovanny Fagua | 2023-08-24 18:30:26 | View | |
14 Dec 2023
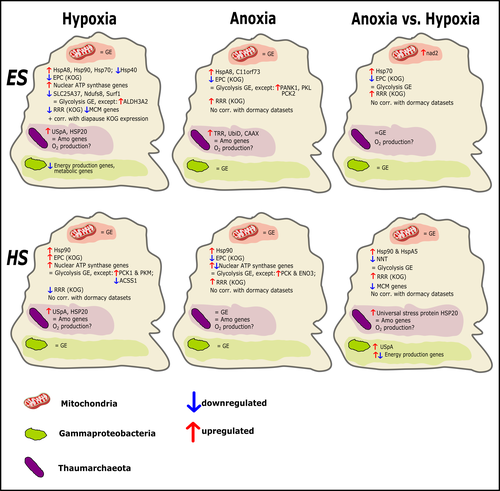
Transcriptomic responses of sponge holobionts to in situ, seasonal anoxia and hypoxiaBrian W Strehlow, Astrid Schuster, Warren R Francis, Lisa Eckford-Soper, Beate Kraft, Rob McAllen, Ronni Nielsen, Susanne Mandrup, Donald E Canfield https://doi.org/10.1101/2023.02.27.530229Future oceanic conditions could leave sponge holobionts breathless – but they won’t let that stop themRecommended by Loïc N. Michel based on reviews by Maria Lopez Acosta and 2 anonymous reviewers based on reviews by Maria Lopez Acosta and 2 anonymous reviewers
It is now widely accepted that anthropogenic climate change is a severe threat to biodiversity, ecosystem function and associated ecosystem services. Assessing the vulnerability of species and predicting their response to future changes has become a priority for environmental biology (Williams et al. 2020). Over the last few decades, oxygen concentrations in both the open ocean and coastal waters have been declining steadily as the result of multiple anthropogenic activities. This global trends towards hypoxia is expected to continue in the future, causing a host of negative effects on marine ecosystems. Oxygen is indeed crucial to many biological processes in the ocean, and its decrease could have strong impacts on biogeochemical cycles, and therefore on marine productivity and biodiversity (Breitburg et al. 2018). Whenever facing such drastic environmental changes, all organisms are expected to have some intrinsic ability to adapt. At shorter than evolutionary timescales, ecological plasticity and the eco-physiological processes that sustain it could constitute important adaptive mechanisms (Williams et al. 2020) Marine sponges seem particularly well-adapted to oxygen deficiency, as some species can survive seasonal anoxia for several months. This paper by Strehlow et al. (2023) examines the mechanisms allowing this exceptional tolerance. Focusing on two species of sponges, they used transcriptomics to assess how gene expression by sponges, by their mitochondria, or by their unique and species-specific microbiome could facilitate this trait. Their results suggest that sponge holobionts maintain metabolic activity under anoxic conditions while displaying shock response, therefore not supporting the hypothesis of sponge dormancy. Furthermore, hypoxia and anoxia seemed to influence gene expression in different ways, highlighting the complexity of sponge response to deoxygenation. As often, their exciting results raise as many questions as they provide answers and pave the way for more research regarding how anoxia tolerance in marine sponges could give them an advantage in future oceanic environmental conditions. References Breitburg et al. (2018): Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240. https://doi.org/10.1126/science.aam7240 Strehlow et al. (2023): Transcriptomic responses of sponge holobionts to in situ, seasonal anoxia and hypoxia. bioRxiv, 2023.02.27.530229, ver. 4 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.02.27.530229 Williams et al. (2008) Towards an Integrated Framework for Assessing the Vulnerability of Species to Climate Change. PLOS Biology 6(12): e325. https://doi.org/10.1371/journal.pbio.0060325 Williams et al. (2020): Research priorities for natural ecosystems in a changing global climate. Global Change Biology 26: 410–416. https://doi.org/10.1111/gcb.14856 | Transcriptomic responses of sponge holobionts to in situ, seasonal anoxia and hypoxia | Brian W Strehlow, Astrid Schuster, Warren R Francis, Lisa Eckford-Soper, Beate Kraft, Rob McAllen, Ronni Nielsen, Susanne Mandrup, Donald E Canfield | <p>Deoxygenation can be fatal for many marine animals; however, some sponge species are tolerant of hypoxia and anoxia. Indeed, two sponge species, <em>Eurypon </em>sp. 2 and <em>Hymeraphia stellifera</em>, survive seasonal anoxia for months at a ... |  | Biology, Ecology, Genetics/Genomics, Invertebrates, Marine, Symbiosis | Loïc N. Michel | Maria Lopez Acosta | 2023-05-12 16:22:47 | View |
14 Nov 2023

Time-course of antipredator behavioral changes induced by the helminth Pomphorhynchus laevis in its intermediate host Gammarus pulex: the switch in manipulation according to parasite developmental stage differs between behaviorsThierry Rigaud, Aude Balourdet, Alexandre Bauer https://doi.org/10.1101/2023.04.25.538244Exploring manipulative strategies of a trophically-transmitted parasite across its ontogenyRecommended by Thierry Lefevre based on reviews by Adèle Mennerat and 1 anonymous reviewerThe intricate relationships between parasites and their hosts often involve a choreography of behavioral changes, with parasites manipulating their hosts in a way that enhances - or seemingly enhances – their transmission (Hughes et al., 2012; Moore, 2002; Poulin, 2010). Host manipulation is increasingly acknowledged as a pervasive adaptive transmission strategy employed by parasites, and as such is one of the most remarkable manifestations of the extended phenotype (Dawkins, 1982). In this laboratory study, Rigaud et al. (2023) delved into the time course of antipredator behavioral modifications induced by the acanthocephalan Pomphorhynchus laevis in its amphipod intermediate host Gammarus pulex. This system has a good foundation of prior knowledge (Bakker et al., 2017; Fayard et al., 2020; Perrot-Minnot et al., 2023), nicely drawn upon for the present work. This parasite orchestrates a switch from predation suppression, during the noninfective phase, to predation enhancement upon maturation. Specifically, G. pulex infected with the non-infective acanthella stage of the parasite can exhibit increased refuge use and reduced activity compared to uninfected individuals (Dianne et al., 2011, 2014), leading to decreased predation by trout (Dianne et al., 2011). In contrast, upon reaching the infective cystacanth stage, the parasite can enhance the susceptibility of its host to trout predation (Dianne et al., 2011). The present work aimed to understand the temporal sequence of these behavioral changes across the entire ontogeny of the parasite. The results confirmed the protective role of P. laevis during the acanthella stage, wherein infected amphipods exhibited heightened refuge use. This protective manipulation, however, became significant only later in the parasite's ontogeny, suggesting a delayed investment strategy, possibly influenced by the extended developmental time of P. laevis. The protective component wanes upon reaching the cystacanth stage, transitioning into an exposure strategy, aligning with theoretical predictions and previous empirical work (Dianne et al., 2011; Parker et al., 2009). The switch was behavior-specific. Unlike the protective behavior, a decline in the amphipod activity rate manifested early in the acanthella stage and persisted throughout development, suggesting potential benefits of reduced activity for the parasite across multiple stages. Furthermore, the findings challenge previous assumptions regarding the condition-dependency of manipulation, revealing that the parasite-induced behavioral changes predominantly occurred in the presence of cues signaling potential predators. Finally, while amphipods infected with acanthella stages displayed survival rates comparable to their uninfected counterparts, increased mortality was observed in those infected with cystacanth stages. Understanding the temporal sequence of host behavioral changes is crucial for deciphering whether it is adaptive to the parasite or not. This study stands out for its meticulous examination of multiple behaviors over the entire ontogeny of the parasite highlighting the complexity and condition-dependent nature of manipulation. The protective-then-expose strategy emerges as a dynamic process, finely tuned to the developmental stages of the parasite and the ecological challenges faced by the host. The delayed emergence of protective behaviors suggests a strategic investment by the parasite, with implications for the host's survival and the parasite's transmission success. The differential impact of infection on refuge use and activity rate further emphasizes the need for a multidimensional approach in studying parasitic manipulation (Fayard et al., 2020). This complexity demands further exploration, particularly in deciphering how trophically-transmitted parasites shape the behavioral landscape of their intermediate hosts and its temporal dynamic (Herbison, 2017; Perrot-Minnot & Cézilly, 2013). As we discover the many subtleties of these parasitic manipulations, new avenues of research are unfolding, promising a deeper understanding of the ecology and evolution of host-parasite interactions. References Bakker, T. C. M., Frommen, J. G., & Thünken, T. (2017). Adaptive parasitic manipulation as exemplified by acanthocephalans. Ethology, 123(11), 779–784. https://doi.org/10.1111/eth.12660 Dawkins, R. (1982). The extended phenotype: The long reach of the gene (Reprinted). Oxford University Press. Dianne, L., Perrot-Minnot, M.-J., Bauer, A., Gaillard, M., Léger, E., & Rigaud, T. (2011). Protection first then facilitation: A manipulative parasite modulates the vulnerability to predation of its intermediate host according to its own developmental stage. Evolution, 65(9), 2692–2698. https://doi.org/10.1111/j.1558-5646.2011.01330.x Dianne, L., Perrot-Minnot, M.-J., Bauer, A., Guvenatam, A., & Rigaud, T. (2014). Parasite-induced alteration of plastic response to predation threat: Increased refuge use but lower food intake in Gammarus pulex infected with the acanothocephalan Pomphorhynchus laevis. International Journal for Parasitology, 44(3–4), 211–216. https://doi.org/10.1016/j.ijpara.2013.11.001 Fayard, M., Dechaume‐Moncharmont, F., Wattier, R., & Perrot‐Minnot, M. (2020). Magnitude and direction of parasite‐induced phenotypic alterations: A meta‐analysis in acanthocephalans. Biological Reviews, 95(5), 1233–1251. https://doi.org/10.1111/brv.12606 Herbison, R. E. H. (2017). Lessons in Mind Control: Trends in Research on the Molecular Mechanisms behind Parasite-Host Behavioral Manipulation. Frontiers in Ecology and Evolution, 5, 102. https://doi.org/10.3389/fevo.2017.00102 Hughes, D. P., Brodeur, J., & Thomas, F. (2012). Host manipulation by parasites. Oxford university press. Moore, J. (2002). Parasites and the behavior of animals. Oxford University Press. Parker, G. A., Ball, M. A., Chubb, J. C., Hammerschmidt, K., & Milinski, M. (2009). When should a trophically transmitted parasite manipulate its host? Evolution, 63(2), 448–458. https://doi.org/10.1111/j.1558-5646.2008.00565.x Perrot-Minnot, M.-J., & Cézilly, F. (2013). Investigating candidate neuromodulatory systems underlying parasitic manipulation: Concepts, limitations and prospects. Journal of Experimental Biology, 216(1), 134–141. https://doi.org/10.1242/jeb.074146 Perrot-Minnot, M.-J., Cozzarolo, C.-S., Amin, O., Barčák, D., Bauer, A., Filipović Marijić, V., García-Varela, M., Servando Hernández-Orts, J., Yen Le, T. T., Nachev, M., Orosová, M., Rigaud, T., Šariri, S., Wattier, R., Reyda, F., & Sures, B. (2023). Hooking the scientific community on thorny-headed worms: Interesting and exciting facts, knowledge gaps and perspectives for research directions on Acanthocephala. Parasite, 30, 23. https://doi.org/10.1051/parasite/2023026 Poulin, R. (2010). Parasite Manipulation of Host Behavior. In Advances in the Study of Behavior (Vol. 41, pp. 151–186). Elsevier. https://doi.org/10.1016/S0065-3454(10)41005-0 Rigaud, T., Balourdet, A., & Bauer, A. (2023). Time-course of antipredator behavioral changes induced by the helminth Pomphorhynchus laevis in its intermediate host Gammarus pulex: The switch in manipulation according to parasite developmental stage differs between behaviors. bioRxiv, ver. 6 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.04.25.538244 | Time-course of antipredator behavioral changes induced by the helminth *Pomphorhynchus laevis* in its intermediate host *Gammarus pulex*: the switch in manipulation according to parasite developmental stage differs between behaviors | Thierry Rigaud, Aude Balourdet, Alexandre Bauer | <p style="text-align: justify;">Many trophically transmitted parasites with complex life cycles manipulate their intermediate host antipredatory defenses in ways facilitating their transmission to final host by predation. Some parasites also prote... |  | Aquatic, Behavior, Crustacea, Invertebrates, Parasitology | Thierry Lefevre | 2023-06-20 15:49:32 | View | |
21 Jun 2023
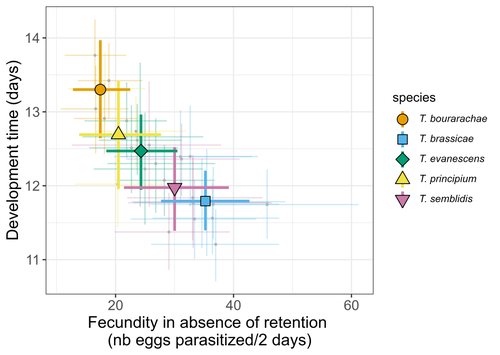
Life-history traits, pace of life and dispersal among and within five species of Trichogramma wasps: a comparative analysisChloé Guicharnaud, Géraldine Groussier, Erwan Beranger, Laurent Lamy, Elodie Vercken, Maxime Dahirel https://doi.org/10.1101/2023.01.24.525360The relationship between dispersal and pace-of-life at different scalesRecommended by Jacques Deere based on reviews by Mélanie Thierry and 1 anonymous reviewerThe sorting of organisms along a fast-slow continuum through correlations between life history traits is a long-standing framework (Stearns 1983) and corresponds to the pace-of-life axis. This axis represents the variation in a continuum of life-history strategies, from fast-reproducing short-lived species to slow-reproducing long-lived species. The pace-of-life axis has been the focus of much research largely in mammals, birds, reptiles and plants but less so in invertebrates (Salguero-Gómez et al. 2016; Araya-Ajoy et al. 2018; Healy et al. 2019; Bakewell et al. 2020). Outcomes from this research have highlighted variation across taxa on this axis and mixed support for, and against, patterns expected of the pace-of-life continuum. Given this, a greater understanding of the variation of the pace-of-life across-, and within, taxa are needed. Indeed, Guicharnard et al. (2023) highlight several points regarding our broader understanding of pace-of-life. In general, invertebrates are poorly represented, the variation of pace-of-life across taxonomic scales is less well understood and the relationship between pace-of-life and dispersal, a key life history, requires more attention. Here, Guicharnard et al. (2023) provide a first attempt at addressing the relationship between dispersal and pace-of-life at different scales. The authors, under controlled conditions, investigated how life-history traits and effective dispersal covary for 28 lines from five species of endoparasitoid wasps from the genus Trichogramma. At the species level negative correlations were found between development time and fecundity, matching pace-of-life axis predictions. Although this correlation was not found to be significant among lines, within species, a similar pattern of a negative correlation was observed. This outcome matches previous findings that consistent pace-of-life axes become more difficult to find at lower taxonomic levels. Unlike the other life-history traits measured, effective dispersal showed no evidence of differences between species or between lines. The authors also found no correlation between effective dispersal and other-life history traits which suggests no dispersal/life-history syndromes in the species investigated. One aspect that was not assessed was the impact of density dependence on pace-of-life and effective dispersal, largely as this was a first step in assessing relationship of dispersal with pace-of-life at different scales. However, the authors do acknowledge the importance of future studies incorporating density dependence and that such studies could potentially lead to more generalizable understanding of pace-of-life and dispersal within Trichogramma. A pleasant addition was the link to potential implications for biocontrol. This addition showed an awareness by the authors of how insights into pace-of-life can have an applied component. The results of the study highlighted that selecting for specific lines of a species, to maximise a trait of interest at the cost of another, may not be as effective as selecting different species when implementing biocontrol. This is especially important as often single, established species used in biocontrol are favoured without consideration of the potential of other species which can lead to more efficient biocontrol. REFERENCES Araya-Ajoy, Y.G., Bolstad, G.H., Brommer, J., Careau, V., Dingemanse, N.J. & Wright, J. (2018). Demographic measures of an individual's "pace of life": fecundity rate, lifespan, generation time, or a composite variable? Behavioral Ecology and Sociobiology, 72, 75. | Life-history traits, pace of life and dispersal among and within five species of *Trichogramma* wasps: a comparative analysis | Chloé Guicharnaud, Géraldine Groussier, Erwan Beranger, Laurent Lamy, Elodie Vercken, Maxime Dahirel | <p>Major traits defining the life history of organisms are often not independent from each other, with most of their variation aligning along key axes such as the pace-of-life axis. We can define a pace-of-life axis structuring reproduction and de... |  | Biology, Ecology, Insecta, Invertebrates, Life histories | Jacques Deere | 2023-01-25 18:15:20 | View |










