
TOUGERON Kévin
- Ecology of Interactions and Global Change lab., Université de Mons, Mons, Belgium
- Biocontrol, Biodiversity, Ecology, Evolution, Insecta, Insectivores, Invertebrates, Parasitology, Pest management, Physiology
- recommender
Recommendation: 1
Reviews: 2
Recommendation: 1

Dose, temperature and formulation shape Metarhizium anisopliae virulence against the oriental fruit fly: lessons for improving on-target control strategies
Optimizing fungal pathogen strategies for oriental fruit fly control
Recommended by Kévin Tougeron based on reviews by François Verheggen and Papa Djibril FayeUsing entomopathogenic fungi for biological control is an effective method for controlling certain crop pests, with the perspective of reducing the use of chemical pesticides. Yet, the efficiency of pathogenic fungi is dependent upon many factors that need to be evaluated to improve biological control potential in the fields (Lacey, 2001). The article by Chailleux et al. (2024) presents an exciting contribution to the field of biological pest control, specifically focusing on using entomopathogenic fungi to manage the oriental fruit fly, Bactrocera dorsalis. This fly, a member of the Tephritidae family, is a major threat to orchards in Asia, the Pacific and Africa, as it attacks fruit and causes considerable damage, in addition to having a relatively rapid biological invasion dynamic (Clarke et al. 2005).
The objective of the Chailleux et al. (2024) study was to evaluate the virulence of Metarhizium anisopliae spores (strain Met69) on B. dorsalis adult flies according to various conditions: the inoculation dose and spore load, the formulation (adjuvant) and temperature conditions. The focus on host specificity and on-target applications was conducted to ensure minimal impact on non-target organisms, which is crucial for sustainable agriculture. The main challenge in this system was to achieve high strain virulence to kill wild individuals with a low number of spores—therefore limiting impact on non-target species such as natural enemies—but with a sufficient incubation period to allow transmission from mass-reared insects to wild conspecifics (Leite et al. 2022). A comparison of different inoculation methods is also provided and is interesting from a methodological point of view for future studies or even large-scale applications.
Using a well-designed experimental setup, the authors show that high pathogenicity (measured by LD50) is achievable even at low spore doses and independently of the fly's sex. Lethal action speed was, however, dependent on the dose. Regarding temperature, the authors demonstrated that mycelium growth was affected by the mean temperature but, most importantly, by daily fluctuation regimes; night and day temperature alternation allowed faster growth than constant temperature. These notions of thermal fluctuations are still under-researched in terms of their modulating role in biological control yet seem central to understanding them, as the authors demonstrate here. The correlation between increased virulence and specific abiotic factors, such as temperature, offers valuable additional insights into the bioecology of the insect host and the fungal pathogen. Chailleux et al. finally point out the need for careful selection of adjuvants in formulations and pay attention to interactions with the abiotic environment to avoid compromising the effectiveness of biological control agents. Indeed, the survival rate of inoculated flies increased in the presence of the corn starch adjuvant, but this effect decreased with temperature. As corn starch unexpectedly delayed mortality, the authors suggest a potential for enhancing conspecific transmission
From a broader perspective, the study emphasizes the importance of standardizing virulence evaluation to optimize biological control strategies like auto-dissemination or vectoring with sterile males, particularly in field conditions. The study contributions are timely and essential for advancing sustainable pest management strategies and improving inoculation methods. The findings underscore the need for field trials to refine these strategies, particularly in Africa, where climatic factors may affect pathogen efficacy and fly behavior. I recommend publishing this article in a referenced journal like the Peer Community Journal.
References
Chailleux, A. Coulibaly, ON, Diouf B, Diop S, Sohel A, Brevault T (2023) Dose, temperature and formulation shape Metarhizium anisopliae virulence against the oriental fruit fly: lessons for improving on-target control strategies. bioRxiv, ver.2 peer-reviewed and recommended by PCI Zoology https://doi.org/10.1101/2023.12.14.571642
Clarke, A. R. et al. (2005). Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol., 50, 293-319. https://doi.org/10.1146/annurev.ento.50.071803.130428
Lacey, L. A. (2001). Formulation of microbial biopesticides: beneficial microorganisms, nematodes and seed treatments. J Invertebr Pathol, 77, 147. https://doi.org/10.1006/jipa.2000.5005
Leite, M. O. et al. (2022). Laboratory risk assessment of three entomopathogenic fungi used for pest control toward social bee pollinators. Microorganisms, 10, 1800. https://doi.org/10.3390/microorganisms10091800
Reviews: 2
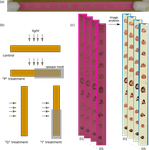
Up and to the light: intra- and interspecific variability of photo- and geo-tactic oviposition preferences in genus Trichogramma
New insights into oviposition preference of 5 Trichogramma species
Recommended by Joël Meunier based on reviews by Kévin Tougeron and Eveline C. VerhulstInsects exhibit a great diversity of life-history traits that often vary not only between species but also between populations of the same species (Flatt and Heyland, 2011). A better understanding of the variation in these traits can be of paramount importance when it comes to species of economic and agricultural interest (Wilby and Thomas, 2002). In particular, the control of the development and expansion of agricultural pests generally requires a good understanding of the parameters that favour the reproduction of these pests and/or the reproduction of the species used to control them (Bianchi et al., 2013; Gäde and Goldsworthy, 2003).
Parasitoid wasps of the genus Trichogramma are a classic example of insects involved in pest control (Smith, 1996). This genus comprises over 200 species worldwide, which have been used to control populations of a wide range of lepidopteran pests since the 1900s (Flanders, 1930; Hassan, 1993). Despite its common use, the egg-laying preference of this genus is only partially known. For example, all Trichogramma species are often thought to have positive phototaxis (or negative geotaxis) (e.g. Brower & Cline, 1984; van Atta et al., 2015), but comprehensive studies simultaneously testing this (or other) parameter among Trichogramma species and populations remain rare.
This is exactly the aim of the present study (Burte et al., 2021). Using a new experimental approach based on automatic image analysis, the authors compared the photo- and geo-tactic oviposition preference among 5 Trichogramma species from 25 populations. Their results first confirm that most Trichogramma species and populations prefer light to shade, and higher to lower positions for oviposition. Interestingly, they also reveal that the levels of preference for light and gravity show inter- and intraspecific variation (probably due to local adaptation to different strata) and that both preferences tend to relax over time.
Overall, this study provides important information for improving the use of Trichogramma species as biological agents. For example, it may help to establish breeding lines adapted to the microhabitat and/or growing parts of plants on which agricultural pests lay eggs most. Similarly, it suggests that the use of multiple strains with different microhabitat selection preferences could lead to better coverage of host plants, as well as a reduction in intraspecific competition in the preferred parts. Finally, this study provides a new methodology to efficiently and automatically study oviposition preferences in Trichogramma, which could be used in other insects with a particularly small size.
References
Bianchi, F. J. J. A., Schellhorn, N. A. and Cunningham, S. A. (2013). Habitat functionality for the ecosystem service of pest control: reproduction and feeding sites of pests and natural enemies. Agricultural and Forest Entomology, 15, 12–23. https://doi.org/10.1111/j.1461-9563.2012.00586.x
Burte V., Perez G., Ayed F., Groussier G., Mailleret L, van Oudenhove L. and Calcagno V. (2021). Up and to the light: intra- and interspecific variability of photo-and geo-tactic oviposition preferences in genus Trichogramma. bioRxiv, 2021.03.30.437671, ver. 4 peer-reviewed and recommended by PCI Zoology. https://doi.org/10.1101/2021.03.30.437671
Brower, J. H. and Cline, L. D. (1984). Response of Trichogramma pretiosum and T. evanescens to Whitelight, Blacklight or NoLight Suction Traps. The Florida Entomologist, 67, 262–268. https://doi.org/10.2307/3493947
Flanders, S. E. (1930). Mass production of egg parasites of the genus Trichogramma. Hilgardia, 4, 465–501. https://doi.org/10.3733/hilg.v04n16p465
Flatt, T. and Heyland, A. (2011). Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199568765.001.0001
Gäde, G. and Goldsworthy, G. J. (2003). Insect peptide hormones: a selective review of their physiology and potential application for pest control. Pest Management Science, 59, 1063–1075. https://doi.org/10.1002/ps.755
Hassan, S. A. (1993). The mass rearing and utilization of Trichogramma to control lepidopterous pests: Achievements and outlook. Pesticide Science, 37, 387–391. https://doi.org/10.1002/ps.2780370412
Smith, S. M. (1996). Biological Control with Trichogramma : Advances, Successes, and Potential of Their Use. Annual Review of Entomology, 41, 375–406. https://doi.org/10.1146/annurev.en.41.010196.002111
van Atta, K. J., Potter, K. A. and Woods, H. A. (2015). Effects of UV-B on Environmental Preference and Egg Parasitization by Trichogramma Wasps (Hymenoptera: Trichogrammatidae). Journal of Entomological Science, 50, 318–325. https://doi.org/10.18474/JES15-09.1
Wilby, A. and Thomas, M. B. (2002). Natural enemy diversity and pest control: patterns of pest emergence with agricultural intensification. Ecology Letters, 5, 353–360. https://doi.org/10.1046/j.1461-0248.2002.00331.x

The open bar is closed: restructuration of a native parasitoid community following successful control of an invasive pest.
Raise and fall of an invasive pest and consequences for native parasitoid communities
Recommended by Stefaniya Kamenova based on reviews by Kévin Tougeron and Miguel González Ximénez de EmbúnHost-parasitoid interactions have been the focus of extensive ecological research for decades. One the of the major reasons is the importance host-parasitoid interactions play for the biological control of crop pests. Parasitoids are the main natural regulators for a large number of economically important pest insects, and in many cases they could be the only viable crop protection strategy. Parasitoids are also integral part of complex food webs whose structure and diversity display large spatio-temporal variations [1-3]. With the increasing globalization of human activities, the generalized spread and establishment of invasive species is a major cause of disruption in local community and food web spatio-temporal dynamics. In particular, the deliberate introduction of non-native parasitoids as part of biological control programs, aiming the suppression of established, and also highly invasive crop pests, is a common practice with potentially significant, yet poorly understood effects on local food web dynamics (e.g. [4]).
In their study, Muru et al. [5] took advantage of an existing biological control program focusing on the Asian chestnut gall wasp Dryocosmus kuriphilus, an invasive (and highly damaging) pest of chestnut trees. The species is currently a successful invader in many geographic regions, including southern France, where local parasitoid communities failed to provide an adequate control since its widespread establishment in 2010 [6]. In response, the non-native parasitoid species Torymus sinensis, which is highly-specific to the Asian chestnut gall wasp, was massively released in commercial chestnut orchards across several regions in France and the island of Corsica. The pest population outbreak was successfully contained, and thanks to the vast amount of host-parasitoid interaction data collected as part of the program, the authors were able to explore the effects of the large fluctuations in Asian chestnut gall wasp natural abundances on native parasitoid communities, immediately before, and up to five years following the introduction of its natural enemy T. sinensis.
Using co-occurrence and clustering analyses, Muru et al. [5] demonstrate that the invasion and the consecutive (efficient) control of the Asian chestnut gall wasp by the parasitoid T. sinensis have a significant impact on the structure of local parasitoid food webs. In particular, following decline in the Asian chestnut gall wasp’s populations, native parasitoids markedly switched to alternative hosts, most likely due to their respectively higher relative abundances. This pattern seemed to be driven by the degree of generalism in native parasitoid species. Indeed, when its abundances were still relatively high, the Asian chestnut gall wasp was primarily attacked by species capable of exploiting a broad range of hosts, while at low population densities only specialist parasitoids such as Mesolobus sericeus were able to persist and compete with the non-native T. sinensis.
The current study is important for two major reasons. First, it underscores the value of long-term species interaction data in order to understand the dynamic nature of food webs, namely their structural flexibility in response to changes in the environment or, as in this case, large fluctuation in abundances of a major pest species. In this context, biological control programs could be a great source of data for exploring long-term, large-scale dynamics of species interactions, and their use in ecological studies deserves to be further emphasized. Second, the study adds to the increasing empirical evidence that mobile generalist foragers can display adaptive, frequency-dependent switching behaviour ([1], [7]), which has been suggested to act as a key stabilizing mechanism in food webs by buffering fluctuating population dynamics at larger spatial scales ([8- 10]).
However, the timing of such buffering seems important, especially in systems such as commercial chestnut orchards. Despite their capacity to adaptively switch their foraging behaviour, the response of the native parasitoid communities to the new, unfamiliar resource was not fast enough in order to contain the primary outbreak under an appropriate damage threshold, thus requiring the introduction of the more specialized parasitoid T. sinensis. Nevertheless, based on current ecological theory, results presented by Muru et al. [5] suggest that the response of native parasitoid community to fluctuating host dynamics – i.e. shifts in parasitoid foraging behaviour based on their traits – could be predictable. This is encouraging considering the growing impact of biological invasions and insect pest outbreaks, but also the need to implement efficient, yet sustainable strategies for crop protection. Future studies would show at what extent observations by Muru et al. [5] are generalizable over longer time periods or other model systems. Noticeably, better understanding about population dynamics and interactions with the broader community of hosts available across habitats should allow to fine-tune predictions about parasitoids’ response to fluctuating resources.
References
[1] Eveleigh ES, McCann KS, McCarthy PC, Pollock SJ, Lucarotti CJ, Morin B, McDougall GA, Strongman DB, Huber JT, Umbanhowar J, Faria LDB (2007). Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 104, 16976-16981. doi: 10.1073/pnas.0704301104
[2] Tylianakis JM, Tscharntke T, Lewis OT (2007). Habitat modification alters the structure of tropical host–parasitoid food webs. Nature 445, 202-205. doi: 10.1038/nature05429
[3] Murakami M, Hirao T, Kasei A (2008). Effects of habitat configuration on host–parasitoid food web structure. Ecol. Res. 23, 1039-1049. doi: 10.1007/s11284-008-0478-0
[4] Geslin B, Gauzens B, Baude M, Dajoz I, Fontaine C, Henry M, Ropars L, Rollin O, Thébault E, Vereecken NJ (2016). Massively introduced managed species and their consequences for plant–pollinator interactions. Adv. Ecol. Res. 57, 147-199. doi: 10.1016/bs.aecr.2016.10.007
[5] Muru D, Borowiec N, Thaon M, Ris N, Viciriuc M I, Warot S, Vercken E (2020) The open bar is closed: restructuration of a native parasitoid community following successful control of an invasive pest. bioRxiv, 2019.12.20.884908, ver. 6 peer-reviewed and recommended by PCI Zoology. doi: 10.1101/2019.12.20.884908
[6] Borowiec N, Thaon M, Brancaccio L, Warot S, Vercken E, Fauvergue X, Ris N, Malausa J-C (2014). Classical biological control against the chestnut gall wasp 'Dryocosmus kuriphilus' (Hymenoptera, Cynipidae) in France. Plant Prot. Q. 29, 7-10.
[7] Bartley TJ, McCann KS, Bieg C, Cazelles K, Granados M, Guzzo MM, MacDougall AS, Tunney TD, McMeans BC (2019). Food web rewiring in a changing world. Nat. Ecol. Evol. 3, 345–354. doi: 10.1038/s41559-018-0772-3
[8] Kondoh M (2003). Foraging adaptation and the relationship between food-web complexity and stability. Science. 299, 1388-1391. doi: 10.1126/science.1079154
[9] McCann KS, Rooney N (2009). The more food webs change, the more they stay the same. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1789-801. doi: 10.1098/rstb.2008.0273
[10] Valdovinos FS, Ramos-Jiliberto R, garay-Narváez L, Urbani P, Dunne JA (2010). Consequences of adaptive behaviour for the structure and dynamics of food webs. Ecol. Lett. 13, 1546-1559. doi: 10.1111/j.1461-0248.2010.01535.x