

Biotic interactions are often shaped by abiotic factors (Liu and Gaines 2022). Although this notion is not new in ecology and evolutionary biology, we are still far from a thorough understanding of how biotic interactions change along abiotic gradients in space and time. This is particularly challenging because abiotic factors can affect organisms and their interactions in multiple – direct or indirect – ways. For example, because abiotic conditions strongly determine how energy enters biological systems via producers, their effects can propagate through entire food webs, from the bottom to the top (O’Connor 2009, Gilbert et al 2019). Understanding how biological diversity - both within and across species - is shaped by the indirect effects of environmental conditions is a timely question as climate change and anthropogenic activities have been altering temperature and water availability across different ecosystems.
Motivated by the current water crisis and severe droughts predicted for the near future worldwide (du Plessis 2019), Migeon et al. (2023) investigated how water limitation on producers scales up to affect life-history patterns of a widespread crop pest, the spider mite Tetranychus urticae. The authors sampled spider mite populations (n = 12) along a striking gradient of climatic conditions (>16 degrees of latitude) in Europe. After letting mites acclimate to lab conditions for several generations, the authors performed a common garden experiment to quantify how the life-history traits of mite populations from different locations respond to drought stress in their host plants.
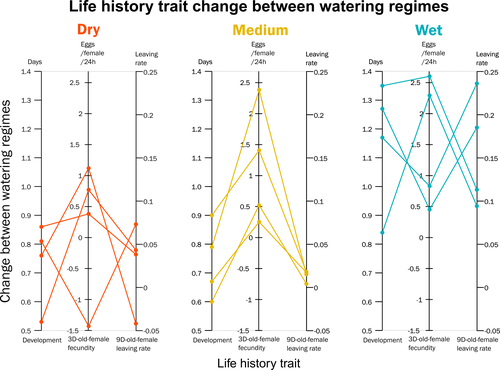
Curiously, the authors found that, when reared on drought-stressed plants, mites tended to develop faster, had higher fecundity and lower dispersion rates. This response was in line with some results obtained previously with Tetranychus species (e.g. Ximénez-Embun et al 2016). Importantly, despite some experimental caveats in the experimental design, which makes it difficult to completely disentangle the specific effects of location vs. environmental noise, results suggest the climate that populations originally experienced was also an important determinant of the plastic response in these herbivores. In fact, populations from wetter and colder regions showed a steeper change in drought response, while populations from arid climates showed a shallower response. This interesting result suggests the importance of intraspecific (between-populations) variation in the response to drought, which might be explained by the climatic heterogeneity in space throughout the evolutionary history of different populations. These results become even more important in our rapidly changing world, highlighting the importance of considering genetic variation (and conditions that generate it) when predicting plastic and evolutionary responses to stressful conditions.
REFERENCES
du Plessis, A. (2019). Current and Future Water Scarcity and Stress. In: Water as an Inescapable Risk. Springer Water. Springer, Cham. https://doi.org/10.1007/978-3-030-03186-2
Gibert, J.P. Temperature directly and indirectly influences food web structure. Sci Rep 9, 5312 (2019). https://doi.org/10.1038/s41598-019-41783-0
Liu, O. R., & Gaines, S. D. (2022). Environmental context dependency in species interactions. Proceedings of the National Academy of Sciences, 119(36), e2118539119. https://doi.org/10.1073/pnas.2118539119
Migeon A., Auger P., Fossati-Gaschignard O., Hufbauer R.A, Miranda M., Zriki G., Navajas M. (2023) The response to drought-stressed host plants varies among herbivorous mite populations from a climate gradient. bioRxiv, 2021.10.21.465244, ver. 4 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2021.10.21.465244
O'Connor, M.I. (2009), Warming strengthens an herbivore-plant interaction. Ecology, 90: 388-398. https://doi.org/10.1890/08-0034.1
Ximénez-Embún, M. G., Ortego, F., & Castañera, P. (2016). Drought-stressed tomato plants trigger bottom-up effects on the invasive Tetranychus evansi. PloS one, 11(1), e0145275. https://doi.org/10.1371/journal.pone.0145275
DOI or URL of the preprint: https://doi.org/10.1101/2021.10.21.465244
Version of the preprint: 3
We appreciate the authors' efforts to make the manuscript more balanced and to communicate the results more clearly and precisely. We would like to emphasize how the current version recognizes the study's methodological limitations, which we strongly believe is a positive aspect. As mentioned in previous reviews, some statistical analyzes could be more elaborate, but the current body of analyzes is not problematic to the point of compromising the interpretation. We would be happy to write a recommendation, after the authors address some minor points raised by the reviewer and ourselves.
Line 46. Maybe a short sentence linking drought to production in field crops is missing here.
Line 119. Replace “Mites” with “Study System” and merge “Origin of mites” in this subsection. The next subsection “Plant material” could be renamed to “Host plant” or simply also included as a paragraph in “Study system”
Line 265. There is a missing period after Experiment I.
Figure 5. Avoid using plot titles in all the panels
DOI or URL of the preprint: https://doi.org/10.1101/2021.10.21.465244
Version of the preprint: 2
The revised manuscript by Midgeon et al was assessed by two Reviewers and ourselves. We all agree that overall it has improved greatly from the previous version.
However, as pointed out by Reviewer 2 there is an important issue that largely reduces the power of several analyses and has not been addressed in the manuscript: the fact that the populations were assayed in different days adds a confounding effect to the population, which prevents comparisons between populations. In addition, this also severely impairs the correct estimation of the correlation between performance and the climatic variables. One possible angle is, as suggested by Reviewer 2 (in the first and second revision), to look at the intraspecific variation in the different populations and how it changes in response to drought stress. Even if the authors do not want to explore this angle, the fact that the populations were assayed in different days and the possible problems that can arise from it needs to be addressed in the results and discussion.
In addition, important information about the methods are missing. In particular, it is key to inform the date of collections, days when populations were assayed and how much time the populations spent in the laboratory (add these informations to the table). This allows the readers to acknowledge that time of collection/day/time in the laboratory can be factors at play in the results obtained.
In addition the material methods do not provide enough information to allow full reproducibility of the statistical analyses. Although we saw that the authors provided the R code and data, information should be also available in the methods section.
Figure 1 - This figure is a bit confusing, since there are three categories, the map should have only three colors (so that it is easy to see which populations belong in each category). This figure with the different colors could then be in supplementary material
Line 85 - intraspecific variation has to be better articulated with the rest of the introduction.
Line 159: 163 - It is not clear what experiment these lines refer to.
Line 282: 284 - It is not clear what was done in this analysis. Were all populations used in the same analyses? or one per population?
The manuscript “Herbivore life histories are altered by drought stress in their host plants” presents the effect of drought on two fitness parameters of mites from different populations charactized by different water regimes. The authors show that mites perform better on water stress plants, and seemed to prefer them over well watered plants. Importantly, they found that the effect of current drought was stronger in mites originating from wetter climate, demonstrating intraspecific variability (and possibly local adaptation) in response to drought.
The second version of the manuscript represents a clear improvement from the previous version. The authors addressed a fair amount of comments made by the three reviewers. In particular, the paper reads much better now, and several minor issues were clarified. However, I still need to be convinced by the modelling approach.
Inferences on the effect of drought on life history traits are based on a series of 12 repeated ANOVA, which is not recommended. This approach not only increases the risk of false discovery, but also prevents testing differences among populations. Yet, this is a key aspect of the paper. A possible alternative would consist in mixed-effect models in which population would be a random factor, crossed with plant identity, as there are two replicates (cotyledons) per plant.
In addition, I have few minor comments:
Review of the manuscript entitled “Herbivore life histories are altered by drought stress in their hosts plants”, a second version of the pre-print “2021.10.21.465244”.
First of all, I apologize to the authors and the recommenders for the delay in this review.
Overall, I found that the authors made a good effort to address most previous suggestions, making this version of the manuscript clearer and more streamlined.
C1: However, in my point of view, the main issue which previously raised my concern (the fact that each population was tested in different moments) was not addressed or even acknowledged. Additionally, I believe that the authors have really interesting results, but I feel that they are underexplored.
As I referred before, I would focus the discussion on the variability of responses observed within populations and why this is important for applied strategies to control this pest, in particular in the face of climate change. I also missed a more thorough link between the results from the different life history traits and the consequences that drought may have on the growth rate of the analysed populations and consequently on the interactions with their host plants. The way I see it, the discussion would gain a lot if sections 1 and 2 were merged. Note that I am not advertising against removing the discussion around the differences in climatic conditions from the collection locations, I would just frame it in the context of the differences observed within each population, instead of across populations. This suggestion is already incorporated in the results section, where the authors show that x out of y populations responded in a given away, but the link with the discussion is, in my view, important and missing.
Other than this I only have a few minor comments regarding this version of the manuscript:
C2: I believe that, in the title, the authors meant “life history traits” instead of “life histories” and “host plant” instead of “hosts plants”.
C3, line 33: I would change “Thus, the climate in the area…” to “… , suggesting that the climate in the area…”
C4: lines 74-76: The link between these two sentences is not very clear, could the authors please rephrase?
C5 line 85: I would say “Interspecific genetic variation”, otherwise intraspecific variation is the differences in response to… not a factor that can modify the response.
C6 line 124: I would remove the “and” after the reference (Migeon et al. 2019)
C7 line 125: This sentence is vague and does not have a reference. I believe that the authors are referring to the sample locations and not the countries as a hole. For example, the majority of Spain in figure 1 has a GAI equal or lower than that of Greece and Cyprus, so it is hard for me to consider it to have an “intermediate” climate. Also, this division is not in accordance with that made in figure S1 where the authors have a location in France and a location in the UK on the “medium climate” category.
C8 line 420-422: the reference is missing.
C9 line 432-434: How do the author results reinforce the optimal balance hypothesis proposed by Alzate et al.? The authors did not measure longevity; the possible link between faster development and higher fecundity does not support the mentioned hypothesis and, as the authors state (lines 425-426), there is no link between early and late fecundity. Could the authors clarify?
C10 460-465: references are missing.
https://doi.org/10.24072/pci.zool.100118.rev22DOI or URL of the preprint: https://doi.org/10.1101/2021.10.21.465244
Version of the preprint: 1
Dear Dr Fragata,
First of all, we would like to thank you and Dr. Raul Costa-Pereira for handling our manuscript, Dr. Bastien Castagneyrol and two anonymous reviewers for their thorough and very valuables comments on our manuscript. We have tried to take into account their comments and suggestions.
We have integrated almost all the suggestions proposed to enhance the manuscript.
We provide responses to reviewers’ comments directly along their comments.
We have also rewritten substantial parts, that we’ll detail below.
Title
According to the suggestions, we have changed the title.
Introduction
The introduction has been shortened, with examples focusing mainly on mites. The last paragraph, introducing the work has also been rewritten to expose more clearly the main focus of our work, namely the link between the variation of the response and the climatic conditions of the locations sampled.
Material and methods
The mites
We added a brief presentation of the mites: biological features, distribution, host plants, development characteristics.
Experimental design
The experimental design has been clarified, few parts moved in supplementary materials.
Climatic variables
The climate variable analysis has been removed to focus on the main purpose, the analysis of life-history traits.
Statistical analysis
That’s the main point. We admit that our design suffers of different lacks. When setting up the experimental design, we have tried to do our best to manage the constraints imposed by the space and time available.
We have changed our ANOVA model which is now Trait ~ Climate (Dryness) * Treatment. We have kept the analysis of correlation between the changes of each trait between the two watering regimes and climate variables.
We have also added the mention of the use of the Shapiro-Wilks and Levene tests for assumptions of the ANOVA which were always respected.
We are aware that our t-test conducted on population to compare the traits on well-watered and drought stressed plants can lead to a greater number of populations with significant differences between the two water regimes. But, in our opinion, it is the way to have a synthetic view of the response for each trait.
Results
As stated above, results regarding the climatic analysis have been removed.
The other parts of the results have been completely changed; figures have been redrawn tables changed or removed. The results are now much more summarized.
Discussion
Partly rewritten and split into two parts and a conclusion to highlight the main experimental results and their possible link with the climatic data of the sampled locations.
Motivated by the current water crisis and severe droughts predicted for the near future worldwide, Migeon et al. investigated how the effects of water limitation on producers scale up to affect life-history patterns of a widespread crop pest, the spider mite Tetranychus urticae. The authors sampled spider mite populations (n = 12) along a striking gradient of climatic conditions (>16 degrees of latitude) in Europe. After letting mites acclimate to lab conditions for several generations, Migeon et al. performed a common garden experiment to quantify how life-history traits of mite populations from different locations respond to drought stress in their host plants.
The manuscript was reviewed by three researchers with a large experience in eco-evo approaches to study insect-plant interactions. Overall, the three reviewers think, and both of us agree, that the manuscript addresses a timely question using an interesting study system. Figures are also clear and appealing; Figure 1 is excellent. However, their assessments also raised concerns about the writing style of the manuscript, methods, statistical analyses, and interpretation of the results. Therefore, we suggest the authors pay careful attention to the valuable comments provided by the three reviewers.
We recommend the authors rework the last paragraph of the introduction to communicate more clearly their a priori predictions on the effects of climate of origin and drought-stress on life history. In addition, we recognize the immense logistic challenges of studying life-history variation across populations, especially considering the spatial scale of the study. However, we can’t help but notice that assaying populations at different moments (line 202) adds a relevant source of noise to the results. As noted by all the reviewers, there is not much to be done about this at this moment, other than acknowledging this issue and interpreting results accordingly. Reviewer 3 presents a suggestion to refocus the article on the intraspecific differences in response to drought stress, but using the intra-population variation, instead of between populations variation to drought response.
The three reviewers pointed out that many sentences of the manuscript are hard to follow because of troublesome grammar or structure. Also, all of them noted that the introduction and discussion could be substantially shortened and streamlined. For instance, the plentiful examples presented along the introduction are informative, but also distract the readers from the central message of the paper. We agree with the reviewers’ assessment and believe that the manuscript would largely benefit from thoughtful proofreading and revision of text structure, not necessarily from an English-native speaker. Minor comments provided by the reviewers are helpful in this sense. Another consensus among reviewers is the need to change the title, which is long indeed, and should mention life history to communicate more clearly the scope of the study. Finally, as mentioned by the reviewers, several of the figures and tables may be moved to supplementary material, so that the readers can better focus on the main results.
Dear Dr Fragata,
Thank you for waiting an extra week before I could send my comments on the paper entitled « Can intraspecific variation in an herbivorous mite alter responses to drought-stressed host plant? A common garden experiment in the context of climate change ».
By comparing key life history traits of mites from 12 populations throughout Europe, the authors found that water stress had an overall positive effect on mite fitness. Interestingly, their results suggest that drought exacerbated inter-population differences in life history traits. There are also evidence for local adaptation of mite populations to climatic conditions.
The authors addressed a nice question, and collected a fair amount of data. However, my general feeling is that the data is underused. The paper is framed in a quite descriptive way. Although decsribing a biological system is always valuable, I believe there is enough material here to make the paper more appealing from an ecological point of view. The introduction and discussions are quite symptomatic of this. I appreciated the fact the authors illustrated their claims with examples, but I felt that often the main idea was diluted by the repetition of examples. I often missed the big question. It was the same with the results section.
Maybe I missed something important, but I understood that the main interest of the study laid in the differential response of different populations to water stress, and in the fact that population-specific response to drought could be explained by climatic conditions in the region populations originated. Statistically speaking, this would be an ANCOVA of the form
Life history trait ~ Drought * Climate
This brings me to another important comment. I understood that populations were tested separately « due to restricted space available » (L202). This surely have important statistical consequences as the effect of « Population » is confounded with the effect of « Date ». But assuming populations were essayed in a random order (which is not explaikned, but should have been), and not in a systematic order such that the effect of time would have been confounded with e.g. the temperature of areas populations originated from, mayvbe that’s not a big deal. Or at least this is acceptable to test the effect of population properly. Please note that I am a bit puzzled with this last comment as on the one hand the authors explain they ran separate models for each population (which is reflected by e.g. Table 2) whereas on the other hand, the provide F values to test the effect of ‘Population’ in Table 3.
Repeating an ANOVA for each population is not a recommended approach because the greater the number of tests, the higher the probability to get a significant effect. Also, by doing so, it is not possible to test the effect of population. A clearer description of models that were used would have been useful, maybe with the equation of the model.
In terms of general presentation, I was not convinced that every tables and figures were necessary. In several instances I will list in detail below, information presented in tables could have been moved to the figures. It is a matter of taste and I am very open to contradiction, but now that raw data are made available together with the paper, I don’t feel that systematically reporting raw means for every population and every trait, or the raw difference between water treatment is useful. Not if it dilutes the reader’s attention and distract her/him from the biggest result.
You surely have noticed that my grammar is sometimes loose so I don’t feel qualified to comment on that aspect but there were some sentences I was not comfortable with.
I hope the authors will find some of these comments useful.
Best regards
Bastien
##
Title – I was confused with the word “intraspecific”, although it is perfectly correct. I am too far from genetics to be comfortable with the concept of local adaptation, and maybe it is not what was tested here, but “local adaptation to climatic conditions influences mite response to drought” could make it. That way one can get read of the second part of the title, which is a bit long, maybe
L61 – ‘Parasite’ is too restrictive, and does not match with ‘pest’ that pops out next line (L62)
L65-89 – This is a very long paragraph that could probably be shortened by referring to synthesis on the effect of drought on herbivory (there are a few meta-analyses on this topic).
L96 – it would be great to tell what is the direction of the effect of the ‘latitudinal gradient’ .
L107 – To me, it is acceptable to assume that there is no need to demonstrate or illustrate that ‘intraspecific variation is common in many organisms’.
L110-111 – This sentence give a very descriptive and a-hypothetical flavour to the paper. Yet it is surely possible to elaborate directional hypotheses, should the theoretical context of the paper be elaborated more in the introduction (see above).
L122 – Why was it important to have green and red forms? The paper has some value even for non-mite people, but they need more information on the model system. Life history traits and their ecological significance are not explained. Generally speaking, I missed a paragraph on the biology of the model species. What is its distribution range. Its host range? What are green and red forms? What are the ecological implications of arrhenotoky or sex ratio in terms of plant-mite interaction?
Table 1 – It is unclear whether ‘host plan name’ is the host plant the population is specialized on, or the plant the initial mites were collected on. That’s not exactly the same. Note that Table 1 is largely redundant with Figure 1. I would report information in table 1 to Figure 1.
L132 – Add ‘GAI’ after ‘Global Aridity Index’
L144 – should ‘to’ be ‘onto’?
L148 – should it be ‘and left THEM oviposit’ ?
L153 – I understood that SP-I population feeds on Phaseolus vulgaris. This mean that save for this population, all other mites had to face an host shift during the experiment. Could it have influenced the outputs? In a perfect world we would have used at least 3 host plant models, but I understand the technical constraints, one have to be realistic. But maybe the authors could mention this issue?
L192 – Were the same 10 plants used each time, or was it a random sample? This has consequences in terms of analyses.
L214 – add a ‘,’ after ‘sowing’?
Figure 3 – I appreciate the authors show there experimental set up. It can be useful for people willing to use the same method (I may well do!), but maybe the figure would be better placed in Sup. Mat. ?
L246 – As for the green vs. red types, it may be good justifying what why essays were conducted on 3- and 9-days individuals. Only when reading the discussion I approximated the rationale.
L262 – Climate of the region populations originated from, or something along that? Also, I did not understood why it was necessary to use two databases. I assume that CGIAR brought something that was missing in worldclim, otherwise it would not have been used, but I don’t know what.
L273 and following – Please refer to my general comment on the modeling approach
L305 – ‘a quite wide range of climatic conditions’ is fairly subjective. For instance, how was this range compared to that experienced by the species throughout its whole distribution range?
L304 – I didn’t feel that this whole section and corresponding analysis was much useful, because if I understood well, coordinates on the PC axes were not used. If this analysis only aimed to roughly classify the populations, then it could have been moved to supp. mat. But maybe it can simply be deleted, with no hurt.
Table 2 – data in columns 2 and 3 don’t seem to have been logit transformed, as mentioned in the caption. Were they?
L433 – I would have started with summary statistics (same for the next sections).
Note that I do not comment much on the results section as what I indicated so far also applies to other subsections.
L509 – That would be great to have an overall statement of “the big result” here. At first, I thought one could say that “drought had a positive effect on mite fitness”, but then I wondered whether if one can interpret the results that way, because there might be a trade off between reduced development time and total fecundity.
L522-524 – ‘development time is an intrinsic parameter…’. I am not sure what to think about this sentence. When the plant is not stressed, there are no population-specific differences in development time. But drought revealed differences, suggesting that there was some kind of genetic differentiation among populations. The current sentence ignores this result, which is an important one. I admit I have difficulties to rephrase the sentence, but I trust the authors will do a great job (or explain me I am wrong, which is just fine too).
L535-539 – The two sentences does not flow. The ‘for instance’ is not appropriate here, as it seems the same idea is repeated.
L541-547 – As there are obviously many differences between the present study and that by Ximenez-Embun, I don’t think this bit is relevant. I would by far prefer that the author bring papers from other systems to generalize their results instead of compare very precisely their number with that of other papers.
L566 – I don’t think that the phrasing ‘the hypothesis tends to…’ is appropriate as the above sentence is not really an hypothesis. Also, because the above sentence is based on the literature, it is quite normal that it is supported by the literature. Maybe consider rephrasing?
L572 – ‘feeding’ instead of ‘exposed to feed’ ?
L579 -580 – I understand the idea, but the present sentence is wrong, as it implicitly suggests that the mites – the individuals – experienced different conditions. This is true for the population, not the individuals.
https://doi.org/10.24072/pci.zool.100118.rev11
Please find below my review to the MS titled: "Can intraspecific variation in an herbivorous mite alter responses to drought-stressed host plant? A common garden experiment in the context of climate change" (2021.10.21.465244v1.full).
First I must say I found quite hard to read the MS as it is, most probably due to English issues. Although it is not my native language I find many sentences are not well written making some ideas not clear. I strongly suggest the author(s) to improve English with the help of a native speaker. Manuscripts like these are so much time consuming.
This research aims to evaluate the role of the geographic origins of mites in their response to climatic dry conditions in four life history traits: development time, fecundity, sex-ratio and emigration rate.
As important comentaries:
- I found the title is incomplete. One does not understand what kind of intraspecific variation on herbivorous mite authors are talking about. I would change it to: "Can intraspecific variation in IN LIFE HISTORY TRAITS OF an herbivorous mite alter responses to drought-stressed host plant? A garden experiment in the context of climate change".
- The MS needs the revision of an expert researcher from their laboratories and from a native English speaker.
- Considering Methodology: these mites come from different places and authors put them in chambers with the same t° and RH for their experiments. They do not explain if the selected t° and RH values represent the average from all the collection places. They need to explain how ad why did they choose these particular values. ± 10 % RH means a range of 50-70 % which I consider it represents a large RH variability that could be representative for mites behavior. How controlled were their experimental chambers is not explained. It is not clear for me either what are the treatments tested here. Finally, I ask myself what would be the consequences of doing so considering mites are naturally selected to different developmnetal conditions (even 6 generations later)? This is not measured. Any literature to support it?
- In the rearing plants methodology, again, ± 20 % RH variability range means plants were reared between a 30-70 % RH which I find enormous! and potentially harmful for the experiment.
- The total number of Tables and Figures in the Manuscript must be reduced / synthesized . Half of them must be chosen as Appendix for Supplementary Material.
- Discussion is quite hard to read. On the one hand I may synthesize important ideas to make them clearer, on the other hand I may go deeper into other ideas such as the role of the different genotypes that could be explaining their results. Then in a Conclusion sub-part I will put some recommendations for further studies.
Please find below a line by line revision of the manuscript.
TITLE
Importantly, I found the title kind of incomplete? One does not understand what kind of intraspecific variation on herbivorous mite you are talking about. I would change it to: "Can intraspecific variation in IN LIFE HISTORY TRAITS OF an herbivorous mite alter responses to drought-stressed host plant? A garden experiment in the context of climate change"
ABSTRACT
L25-L29: Difficult to understand. For example, what is the relationship between arthropods' bigger offspring and faster development with attractiveness. Furthermore, of what kind of attractiveness are you talking about? Don't get it.
L31: What do you mean with: "depending on the climatic conditions of the localities at origin"? I don't think "depending" is the correct verb? Which localities at origin are you talking about?
L31-L37: Needs English review by a native speaker. I am not sure if I completely understood the message here.
L38: What do you mean with "leaf patches"? This must be explained somewhere before.
L39: What does "respond more strongly" means? More strongly on displaying shorter developmental time? attempting to leave leaf patches less often? young females more fecund? sex ratio? emigration rate? All of them? Yo must be explicit.
L41: Replace "aridity values" by "aridity levels" or "aridity ranges".
L41-L42: Results actually indicates that mite feeding behaviour contrasts with the climatic conditions they faced in the area of origin, isn't it? If I understood well.
KEYWORDS
L45: KEYWORDS, in plural
L47: "common garden" what does that mean? Backyard garden?
L47-L48: Please put keywords in alphabetical order.
INTRODUCTION
L52: I may replace "that was the title of an article" by "warned The New York Times magazine in ..."
L52: Now, why use this article having hundreds of scientific appers telling the same thing? I would understand if it is something unique from investigation journalism, but this is not the case.
L54: REFs at the end of the sentence.
L56: REFs at the end of the sentence.
L56-L57: Put Mitlin et al. 2019 at the end of the sentence.
L60: Water use will increase how much? Need numbers here, and REFs supporting them.
L62: More REFs here?
L63: REFs at the end of the sentence.
L66-L67: How drought affects plants physiology?
L68: What kind of changes in the amino-acids and free sugar balances were found in drought-stressed plants?
L943: "as a main way"
L96: "... localities." Period missing here.
L107: I would be more modest. I would say "...intraspecific variation seems to be common for many many organisms in drought conditions"
MATERIAL AND METHODS
L118-L119: I may put this sentence at the beginning of this paragraph, start with the main actor.
L121: Sounds weird.
L122: OK, those green and red forms must be described in the Introduction. This is the first time you mention it. And moreover, what is the differene bewteen both in terms of physiology, metabolism etc?
L126: medium hot or medium wet? Please explain.
L142-L143: These mites come from different places and you put them in chambers with the same t° and RH. Are these average values from all the collection places? How and why did you choose these particular values? ± 10 % RH means a range of 50-70 % which I consider it represents a large RH variability that could be representative for their behavior. What are the treatments tested here? This is not clear for me. And finally, what would be the consequences of doing so considering they are naturally selected to different developmnetal conditions (even 6 generations later)? How do you measure this? Any literature to support this?
L144-L150: Why not to explain all this directly here? It would be easier to understand at once.
L158_ Seeding time? Sorry don't inderstand this? Seedling?
L166: Again, ± 20 % RH means a variability range of 30-70 % RH which I find enormous! and potentially harmful for the experiment.
L202-L205: Redundant. Delete. This was already said.
L257-L259: How many generations in total?
L262: "World"
L286: Does the residuals followed a normal distribution in order to use this parametric test?
L297: Does the residuals followed a normal distribution in order to use this parametric test? ... and date meet at least half the ANOVAs assumptions? Particularly
homoscedasticity of the variances.
RESULTS
L306: Where can you say they develop the better?
L316: OK, but I don't get the idea of taking mites from all these places to put them in one "same" t° and RH chamber, and test up to 6 generations the effect of drought in their life history. Based on the title of your work you expect variability from genotypes?
L412: Replace "grater" by "larger"
DISCUSSION
Discussion is quite hard to read. On the one hand I may synthesize important ideas to make them clearer, on the other hand I may go deeper into other ideas such as the role of the genotypes that could be explaining your results. Then in a Conclusion sub-part I will put some recommendations for further studies.
L509-L510: What? Rephrase.
L520-L522: Don't understand this first part of the sentence. Rephrase.
L518-L532: Very hard to read and understand the central points. Try to syntesize the ideas here.
L542: "seen by" not "seen in"
L581-L589: This sounds interesting. Could you develop the mechanisms here?
L594: "for an example"? You mean "for example"?
TABLES AND FIGURES
You will be asked to reduce / synthesize the total number of Tables and Figures in your MS. Half of them should be chosen as Appendix for Supplementary Material.
Table 1 lacks acronyms explanations. Please fill that in the legend.
Figure 1 is interesting indeed, however the italian spot does not represent what in L125 says: "correspond to places with dry hot summers" which can be seen for the rest of the Country. In the end you have mites from 6 vs 4 different kind of summers (and not 5 vs 5 as it pretended to be). Is this important for your treatments?
Figure 3. Legend lacks information about the picture's elements. It might not be considered for the main MS but as an Appendix in SM instead.
Table 2 legend lacks information. Please fill it.
Figure 5: Nice fig.
https://doi.org/10.24072/pci.zool.100118.rev12Review of the pre-print entitled: “Can intraspecific variation in an herbivorous mite alter responses to drought-stressed host plant? A common garden experiment in the context of climate change”
In this manuscript, the authors characterized the intraspecific variability in the response of an herbivorous mite to drought-stressed host plants and aimed to assess if climatic differences in the geographic origin of mite populations explained the variability in their response. This was performed by sampling mite populations on different locations of a climatic gradient and, after 6 generations of acclimation to laboratory conditions, testing life-history traits of such populations on drought-stressed and control bean plants. The authors also assessed differences in dispersal attempts of all populations from drought-stressed and control bean plants.
Climate change affects plant-herbivore interactions, via changes in temperature, extreme drought-events, among other factors. Such effects may have important consequences for the management of crop production and control of crop pests. Recent work from several authors has focused on the effects of drought on different plant-herbivore systems, including herbivorous mites. Here, the authors, add relevant knowledge on intraspecific variability for the response of herbivores to drought-stressed plants, assessing herbivore populations that were sampled in a climatic gradient having, therefore, experienced differently the effects of climate change.
The main short coming of this work, in my view, is that each population was assessed in separate moments (line 202), being impossible to disentangle if the observed differences among populations are derived from their genetic background, which may be linked to the climatic characteristics of their sample location, or from confounding effects pertaining from uncontrolled/unidentified differences between the experimental blocks. I understand the logistic limitations of performing a study with this number of field-collected populations, however this issue could have been solved if the 12-15 replicates per experimental treatment of each population were divided among experimental blocks containing many populations.
Nevertheless, and being impossible to tackle this issue à posteriori, in my point of view, the information provided by the differences in life-history traits between drought-stressed and control plants for each population is very relevant for this research area. Considering intraspecific variability in the response of herbivores, whatever the cause, is key to the development of pest control strategies and to understand and predict the effect of climate change on plant-herbivore interactions in general. With this in mind, I highly suggest that the authors focus the scope of this manuscript on these intra-population differences, keeping the discussion of link between the differences in climate among the geographic locations of the samplings and the observed differences in life-history traits of this herbivorous mite as a possibility.
Another issue regarding the analyses of the results is that on experiment II the authors used 3-day old and 9-day old females to assess life-history traits. Even though, as I understood, both type of females were used on drought-stressed and control plants, they were used in different experimental blocks (line 246). If this is the case, I believe that is important to present the result for 3-day old females and 9-day old females (as the authors did) to show other types of intraspecific variability. However, I would not compare the results from females with different ages. If I understood it wrong, and the 2 batches of plants (referred to in line 246) were used at the same time, please clarify this in the text and ignore the rest of this comment.
Other then these two main issues regarding the analyses of the results I only have a few minor comments that I mention below:
C1: Regarding the title: In my view it is not intraspecific variation that alters the response of herbivores to drought-stressed host plants. I think that the question is “Is there intraspecific variation for…”
C2: Line 26-28 I don’t understand this “…but attractiveness can also occur”. Attractiveness of the herbivore offspring? Of the plants? Could the authors please clarify?
C3: In my view the first paragraph is too long and not directly linked to the main message of the manuscript. The sentences between line 52 and 60 could be summarized in one sentence.
C4: line 107. This sentence is very broad, yes intraspecific variation is common in many organisms. Can the authors specify and maybe link this sentence to the previous paragraph?
C5: table 2: Df is not reference is degrees of freedom, please clarify this in the legend of the table. And also, where do the 8 degrees of freedom come from? Weren’t there 12 to 15 replicates? (line 225)
Download the review https://doi.org/10.24072/pci.zool.100118.rev13