
What gets left behind? Shared nematode communities at the wildlife-livestock interface.
 based on reviews by 2 anonymous reviewers
based on reviews by 2 anonymous reviewers
Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheep
Abstract
Recommendation: posted 30 July 2024, validated 31 July 2024
McCoy, K. (2024) What gets left behind? Shared nematode communities at the wildlife-livestock interface. . Peer Community in Zoology, 100248. 10.24072/pci.zool.100248
Recommendation
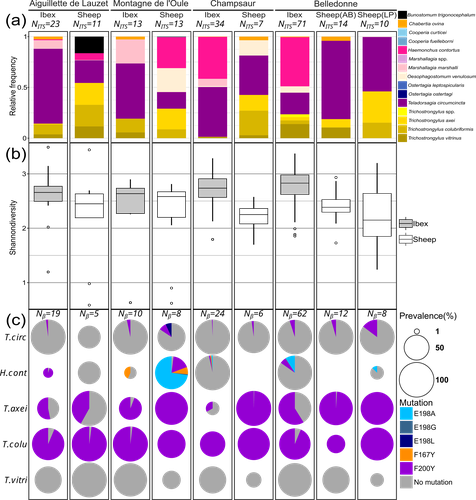
Gastrointestinal nematodes represent a major problem for livestock production across the globe, one that has intensified with the rapid and repeated evolution of multi-drug resistance (Wit et al., 2021). Understanding parasite exposure and how resistance is maintained over time are therefore of key importance for defining efficient management strategies. To date, the role wildlife play in these dynamics has been poorly studied. The work of Beaumelle et al. examine this essential question by studying the transmission dynamics of nematodes at the environmental interface between transhumant sheep and wild ungulates, more specifically with ibex (Capra ibex) that allochronically share alpine pastures when sheep are brought to graze in summer. By collecting fresh fecal material from both species and using a metabarcoding approach based on ITS-2 sequences, the authors characterise the nemabiome in each ungulate species and demonstrate that the two host species share a large portion of their parasite diversity. More importantly, by focusing on a gene (β-tubulin isotype 1) associated with resistance to a commonly used anthelmintic drug (benzimidazole), they demonstrate that both species carry resistant nematode strains, but that the diversity of strains, and particularly susceptible strains, is much higher in ibex. A key feature of the sampling design is that fecal material from both species was collected before seasonal transmission between the ungulate species could occur. Therefore, their results demonstrate that ibex are able to maintain resistant strains over long periods of time and therefore may be major nematode reservoirs for sheep infection. This important conclusion raises a series of key questions. How are resistant genotypes maintained in untreated ibex hosts? Is the cost of resistance so weak that they can coexist with susceptible strains in the absence of drug treatment or does anthelminthic contamination of the pastures maintain resistant genotypes directly in wild hosts? This work also opens several interesting perspectives: For example, what additional resistant parasites may be maintained by these wildlife hosts? What role do other wild ungulate species play in the evolution of nematode communities in transhumant sheep? An expansion of this work to the larger community of wild ungulates using alpine pastures, and an evaluation of the degree to which wild species are exposed to anthelminthic drugs released by grazing livestock into the environment is now required to understand the deeper consequences of drug treatment for shaping parasite communities and their cascading impacts for wildlife conservation, and the development of efficient and sustainable management strategies for pastoral livestock.
References
Beaumelle et al. Cross-transmission of resistant gastrointestinal nematodes between wildlife and transhumant sheep. bioRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Zoology. https://doi.org/10.1101/2023.07.21.550073
Wit, J., Dilks, C.M., Andersen, E.C., 2021. Complementary Approaches with Free-living and Parasitic Nematodes to Understanding Anthelmintic Resistance. Trends Parasitol. 37, 240–250. https://doi.org/10.1016/j.pt.2020.11.008
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
This project was founded by the Office Français de la Biodiversité, the Laboratoire d’Ecologie Alpine (LECA) and VetAgro Sup - Pôle d’Expertise Vétérinaire et Agronomique des Animaux Sauvages (EVAAS, France; http://evaas.vetagro‑sup.fr/; DGAL—VetAgro Sup - INRAE funding). G. Bourgoin was supported by the AgreenSkills+ fellowship program (European Union program; MarieCurie FP7 COFUND People Programme; grant agreement n_609398).
Evaluation round #3
DOI or URL of the preprint: https://doi.org/10.1101/2023.07.21.550073
Version of the preprint: 4
Author's Reply, 22 Jul 2024
Dear Karen McCoy,
We thank you for your thorough review of the manuscript. We have also corrected additional minor errors. Please see the tracked changes in the attached files.
Best wishes,
Camille Beaumelle, on behalf of all co-authors
Decision by Karen D McCoy , posted 04 Jul 2024, validated 04 Jul 2024
, posted 04 Jul 2024, validated 04 Jul 2024
Dear authors,
Thank you for this version 4. The revisions are fine, but I found some errors while reading it over again (sorry I didn’t catch them in the last revision). Most are only minor editorial mistakes, but those of lines 423 and 494 are a little more important and it would be worthwhile to correct them. I have started to write the recommendation, so as soon as the new version is resubmitted, the recommendation text should be ready to post.
Line 101: ‘living’ not ‘leaving’
Line 276: Change to ‘centrifuged’
Line 278: Change to ‘..transferred onto a…’
Line 290: Change to ‘…a Qubit..’
Line 389: Change to ‘..collected. We considered…’
Line 423: Here, you only talk about 3 sites, whereas previously you referred to 4 sites. This is confusing.
Line 462: Change to ‘…The parasites…’
Line 494: This is the first appearance of the acronym RRA and it is only defined in Table 5. This measure should be mentioned in the methods as you actually never define how you measured ‘abundance’ (maybe in the part Statistical analyses on measures of nemabiomes).
Line 505: Explain that you are only talking about ibex here.
Line 510: Change to ‘…presented…’
Line 511: There is an extra bracket that needs to be removed
Line 534: Delete the comma after the word ‘deleted’
Line 552: Change to ‘where’
Line 686: Delete the word ‘the’ before the word ‘testosterone’
Line 694: Delete the word ‘However’.
Line 696: Change to ‘…exchange of parasites…’
Line 710: Remove the ‘s’ on the word ‘parasites’
Line 717: Change to ‘… and molecule degradation can last days, or…’
Line 741: Change to ‘…their life history…
Lines 746,755, 756: Put species names in italics
Line 757: Add a ‘s’ to the word ‘allele’
Line 768: Delete the word ‘had’
Line 772: Add a ‘s’ to the word ‘anthelmintic’
Line 773: Change to ‘…and the fitness cost…’
Line 788 : You should maybe give this in the conditional since previously in the discussion you mention that anthelminthics in the environment could maintain resistant strains. (eg., As parasites on pastures may be less subject to selection pressure by anthelmintics, they could be a source of susceptible strains)
Line 800: ‘in’ ibex
Line 824: remove ‘in Belledonne’
Line 827: change to ‘However, it is possible that this observation…’
Line 828-831: This last sentence should be deleted as the idea is not developed.
Lines 853-856: This sentence could be removed as it repeats the same idea as the preceding sentence.
Evaluation round #2
DOI or URL of the preprint: https://doi.org/10.1101/2023.07.21.550073
Version of the preprint: 2
Author's Reply, 21 Jun 2024
Decision by Karen D McCoy , posted 06 Apr 2024, validated 08 Apr 2024
, posted 06 Apr 2024, validated 08 Apr 2024
Dear authors,
Thank you for the effort that you put into the revision of your manuscript; it is much improved and merits recommendation. However, before I can do so, some further minor revisions are required.
- line 248: the method described here is very difficult to understand and requires some clarification
- line 387: I don't believe that you justify separating the two sites at Cerces; you go from talking about 3 lcoations to 4 locations.
- Figure 3: I think that there is a problem with the haplotype colours for T. colubriformis
- line 683: It is not obvious why males are 'certainly more susceptible to parasitism'. This is should expanding on slightly.
- line 700: A clarification of the notion of 'indirect transmission' is required
- line 726: I wonder whether a viable alternative hypothesis might be that antihelminthic drug residues in the environment are high enough to maintain resistance in ibex?
- There are many minor english errors that require revision throughout the manuscript.
These different remarks, along with english suggestions, are embedded directly in the pdf of the revised version as comments.
Best wishes,
Karen McCoy
Download recommender's annotationsEvaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2023.07.21.550073
Version of the preprint: 1
Author's Reply, 20 Mar 2024
Decision by Karen D McCoy , posted 14 Nov 2023, validated 14 Nov 2023
, posted 14 Nov 2023, validated 14 Nov 2023
I have carefully read both the manuscript and the reviewers reports and agree that this work is orginal and contributes to our understanding of parasite transmission at the domestic/wildlife interface and the circulation of drug resistance. In addition to the reviewers remarks, which I found well-justified, the authors should also mention some elements of the nematode life cycle more explicitly (for example, how long do worms live within a host?). In addition, some discussion on possible facilitation/exclusion dynamics should be considered. For example, could infection by one species of nematode in a host individual exclude infection by another? This could be particularly important to consider in terms of resistant strains - if susceptable strains outcompete resistant strains in the absence of drugs, susceptible strains could prevent infection by resistant strains post-exposure. Finally, I have made a series of detailed remarks directly on the manuscript to correct english and awkward sentences; please go over these carefully. There are also some additional content remarks which should be considered during revision.
Download recommender's annotationsReviewed by anonymous reviewer 2, 07 Nov 2023
This is a well-conceived study and has been conducted using appropriate methods. The conclusion is sufficiently supported by the data.
I query the use of diversity metrics since none of the hypotheses concern diversity per se, rather the extent of overlap in parasite species and resistance genotypes between hosts. But this is a minor point and could be addressed by including an a priori hypothesis on diversity (this could be two-tailed to avoid retro-fitting expectation to data).
The introduction is well-written and comprehensive; could perhaps be abbreviated a little (e.g. the section on BZ-resistance history and mechanisms is not too necessary here). Methods, results and discussion are clear.
With regards to the likely spill-over and maintenance of resistant worms in ibex, originating from sheep, could it not also be possible that anthelmintic residues in the environment might lead to exposure of ibex worms and selection in situ?
Overall the study fails to conclude on the extent to which these worm populations are shared between hosts and the role of ibex in maintaining them and influencing genetic composition. This is sensible and justified and the authors are right to be cautious. All the same they might suggest more concretely what further studies are needed to answer this question – especially what is possible regarding longitudinal and intervention studies.
The English is generally excellent but could be improved slightly in places, e.g. typos / spelling lines 44, 138, 146, 153, 163, (non-exhaustive list).
Reviewed by anonymous reviewer 1, 16 Oct 2023
In my opinion, the work is original and the manuscript is well written. The authors question the interface between domestic and wild ungulates and its consequences in terms of parasite transmission in mountain ecosystems, with issues for both pastoral activities and wildlife conservation. Overall, the description of the methods and the results are clear, with sufficient details and useful illustrations; the discussion is interesting and the conclusions are adequately supported by the results. I have only minor comments and some suggestions mainly to enrich the discussion.
Line 57: the results show that Ibex populations harbor resistant strains before the arrival of the sheep, showing that these strains were maintained for a year. However, there is no demonstration that this maintenance can last more than a year in the absence of sheep. The possible contribution of other wild ungulates that potentially frequent the pastures (as chamois) to the maintenance of the resistant strains has not be studied in the present works. Also, and given that the term reservoir refers to a capacity to maintain a pathogen over the long term without external input, I suggest to be more careful in the use of terms. My suggestion would be to reformulate with “and then could act as refuge or even contribute to maintain resistant GIN”. This comment also lead me to a question: is there any data, even in livestock, on the maintenance of the resistant strains in absence of any selection pressure (i.e. absence of the use of anthelminthics)? In my point of view, it would be interesting to discuss this point.
Line 81: the authors introduce the fact that resistances to several families of anthelmintics have been observed but their works focus only on benzimidazole resistance. It would be interesting to explain, in the introduction or the discussion, whether similar studies but for other anthelmintics would be possible and if not to explain why.
Line 176: the acronym ASV is used here for the first time in the manuscript, please explain it.
Line 194 and following: to better understand the extent of the interfaces which are studied and in what proportion these interfaces are explored in the works, it would be useful to provide elements on the extent area of the massifs and that of the sampling locations where feces were collected (if data available).
In this Material and method section, I suggest gathering all the descriptive data on study sites in a table to make their vizualisation easier and to quickly identify differences between sites.
Line 214: please specify whether the term “individuals” concerns all age groups or only adults.
Line 219 and 222: the authors mention the presence of a goats on some pastures, but there is no further mention of goats afterwards. Since Ibex is phylogenetically closer to goat than to sheep, and that the parasitism of goats can differ from that of sheep, qualitatively and quantitatively, I would have found interesting to also explore the parasitism of the latters. I imagine that this was not done for understandable practical reasons, but I suggest that the authors address this point in the discussion (possible impact of the presence of goats on the nemabiome of Ibex?) in a paragraph that I suggest to add to discuss the possible role of other species (see my last comment).
Line 234: the authors explain that, when possible, they collected Ibex feces just after their deposit and in certain cases also during capture. In one of the files provided in appendix (Sample_description_Sheep_ivbex_all.csv), we can see that the data on the sex was registered during capture. Would it be possible to test a possible influence of sex on the results? As the use of pastures by males and females is not similar in space and time for Ibex, one hypothesis could be that the nemabiome are different. In the same way, for feces collected shortly after their emission, would it have been possible to note whether these feces was emitted by groups of males or females to test whether it affects the diversity of gastrointestinal nematode and the anthelmintic resistance?
Line 246/ table1: it is surprising that, in Belledonne, Ibex samples from 2018 and 2019 have been grouped together, without details on the number collected per year, any demonstration that the nemabiome composition is similar from one year to the next, and specification whether the pastoral pressure was similar from one year to the next (same flock size and composition?). This choice to group samples is all the more surprising as some feces were collected in July (in 2018) when one of the objectives of the work was to study parasitic diversity and resistance to anthelmintics before the arrival of the sheep (unless Belledonne is an exception, sheep are usually present on pastures since June). Finally, I did not understand why in the spreadsheet in Appendix there are 93 Ibex while table 1 indicates 80 Ibex samples in Belldone. This may be a misreading on my part, but I haven't seen any explanation of this difference. I have not compared data in detail for all the sites but it would be good to check the concordance of the numbers between the table and spreadsheet, or to explain why the data differ. In the same way, for Cerces MO, unless I am mistaken, the spreadsheet in appendix mention ibex samples in May 2019 but not in 2018, contrary to table 1.
Line 539: it would be useful if the authors clarified that Ibex populations use areas grazed by sheep but most on the time at different times (months of the year or times of the day).
Line 543: the circulation of a pathogen being a prerequisite for its maintenance, I suggest to reverse circulation and maintenance in the sentence.
Finally, I would have found useful to add a point of discussion on the interest of exploring more widely the hosts possibly involved in the transmission and maintenance of nematodes, such as goats (see above) but also of other wild ungulates. The chamois is mentioned in line 122 but not later, although its role could also be questioned (densities sometimes high, spatio-temporal use of pastures different from that of ibex populations and certainly variable between sites).










